おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
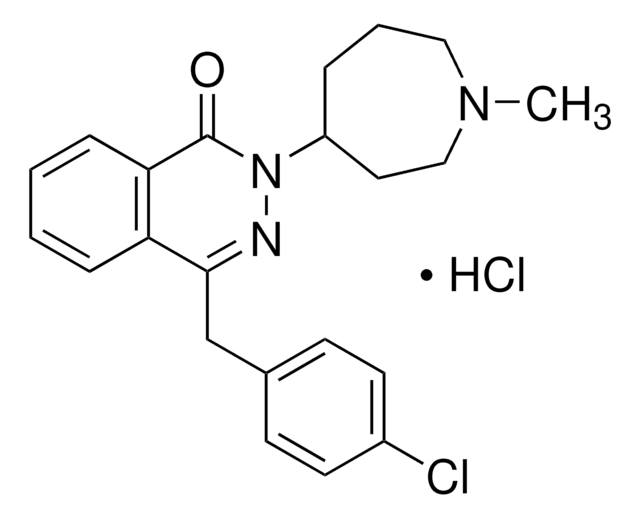
azelastine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
Cl.CN1CCCC(CC1)N2N=C(Cc3ccc(Cl)cc3)c4ccccc4C2=O
InChI
1S/C22H24ClN3O.ClH/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16;/h2-3,6-11,18H,4-5,12-15H2,1H3;1H
InChI Key
YEJAJYAHJQIWNU-UHFFFAOYSA-N
遺伝子情報
human ... HRH1(3269)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Azelastine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生物化学的/生理学的作用
H1ヒスタミン受容体のアンタゴニストであり、NF-kBアクチベーターです。
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Eli O Meltzer et al.
Allergy and asthma proceedings, 33(4), 324-332 (2012-08-04)
Many patients with allergic rhinitis (AR) have uncontrolled symptoms despite available treatment options. This study was designed to evaluate the efficacy and safety of MP29-02 (a novel intranasal formulation of fluticasone propionate [FP] and azelastine [AZ] hydrochloride), compared with monotherapy
Tsugunobu Andoh et al.
Journal of pharmacological sciences, 114(3), 292-297 (2010-10-16)
Recently, we showed that a methanol extract of Ganoderma lucidum inhibits scratching, an itch-related response, induced by intradermal injections of some pruritogens in mice. The present study investigated whether G. lucidum extract would inhibit allergic itch. In mice sensitized with
Carsten Gründemann et al.
Journal of ethnopharmacology, 145(1), 118-126 (2012-11-13)
Extracts from Veronica officinalis L. are traditionally used for the treatment of lung diseases; however, the effective compounds and the mode of action are still unknown. Here we analyzed the effects of a standardized Veronica extract on genes expression and
Hartmut Derendorf et al.
British journal of clinical pharmacology, 74(1), 125-133 (2012-02-24)
• The topical second generation anti-histamine azelastine hydrochloride (AZE) and the potent corticosteroid fluticasone propionate (FP) are well established first-line treatments in allergic rhinitis (AR). • MP29-02, a novel intranasal AZE and FP formulation, has been shown to control AR
Jonathan A Bernstein
Current medical research and opinion, 23(10), 2441-2452 (2007-08-29)
Azelastine hydrochloride (Astelin) nasal spray 0.1% solution is a second-generation intranasal antihistamine available in the US for treatment of both seasonal allergic rhinitis (SAR) and nonallergic vasomotor rhinitis (VMR). Searches of journal articles including the title word 'azelastine' from 1979
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)