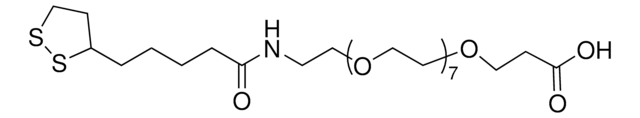
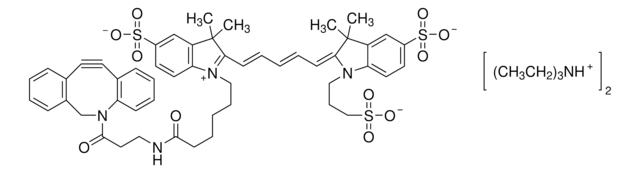
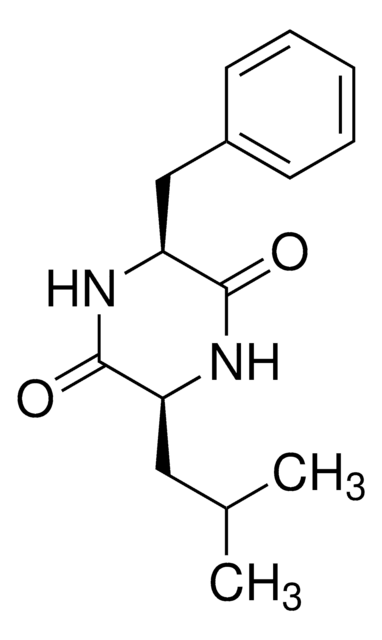
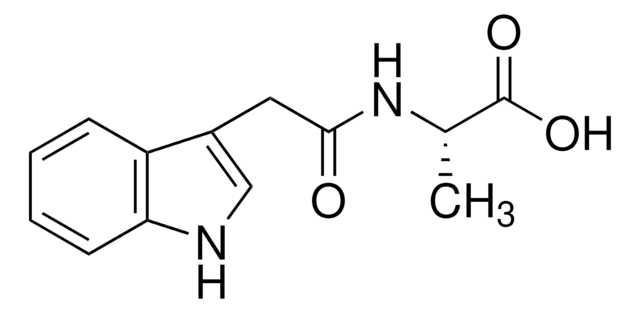
おすすめの製品
詳細
Natural product derived from fungal source.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Eye Dam. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SMB00019-1MG:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
Lot/Batch Number
W S Garcez et al.
Journal of agricultural and food chemistry, 48(8), 3662-3665 (2000-08-24)
Two saprophytic fungi (Mucor ramosissimus and Rhizopus sp.) were tested for their ability to induce phytoalexin production by seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. Only M. ramosissimus was shown to elicit
P H Yu et al.
Canadian journal of biochemistry, 57(10), 1204-1209 (1979-10-01)
The N-acylation of tyramine isomers and other biogenic amines has been studied. The liver exhibits the highest activity towards tyramines, while the brain exhibits a low but significant activity. In the brain, tyramine N-acylation activity was heterogenously distributed. The arylamine
S Friström et al.
Acta pharmacologica et toxicologica, 40(2), 247-258 (1977-02-01)
The effect of five sympathomimetic amines and some of their acetyl derivatives on the blood pressure of the rat was determined on the left carotid artery. After pretreatment with chlorisondamine (1 mg/kg subcutaneously) the blood pressure rise by sympathomimetic amines
A K Mir et al.
Journal of neurochemistry, 36(2), 441-446 (1981-02-01)
N-Acetyltyramine, N-acetyldopamine and N-acetyloctopamine were the major products when either L-[3H]tyrosine or [3H]tyramine were incubated with thoracic ganglia of the desert locust, Schistocerca gregaria. No label was incorporated into L-DOPA under these conditions, although 2-3% of the radioactivity could be
Reversal of resistance by N-acetyltyramine or N-acetyl-2-phenylethylamine in doxorubicin-resistant leukemia P388 cells.
S Kunimoto et al.
The Journal of antibiotics, 40(11), 1651-1652 (1987-11-01)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)