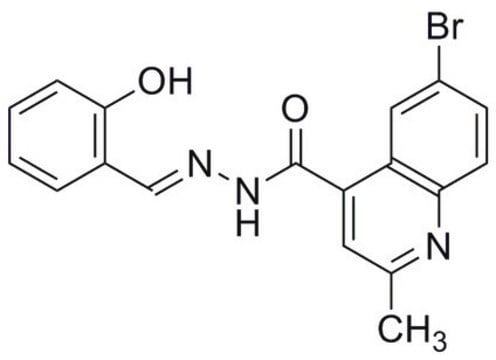
おすすめの製品
アッセイ
≥98% (HPLC)
形状
powder
色
white to beige
溶解性
DMSO: 2 mg/mL, clear
保管温度
2-8°C
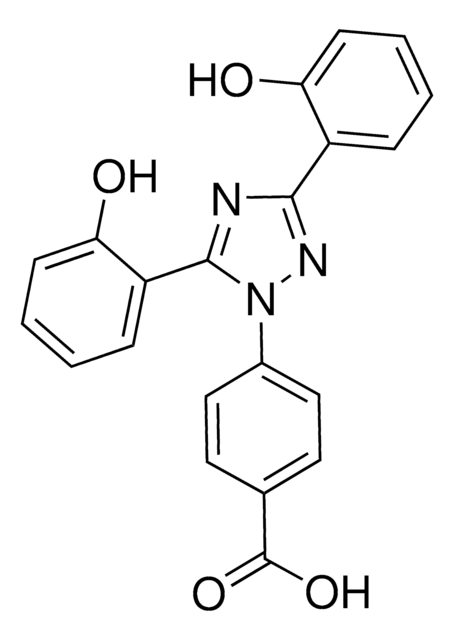
InChI
1S/C21H15N3O4/c25-17-7-3-1-5-15(17)19-22-20(16-6-2-4-8-18(16)26)24(23-19)14-11-9-13(10-12-14)21(27)28/h1-12,22-23H,(H,27,28)/b19-15-,20-16+
InChI Key
FMSOAWSKCWYLBB-VBGLAJCLSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
Deferasirox has been used as an iron chelator to test its effect on clofazimine mediated growth inhibition and rescue in Salmonella typhimurium.
生物化学的/生理学的作用
Deferasirox belongs to the N-substituted bis-hydroxyphenyl-triazole family of tridentate iron chelators.
Deferasirox is an orally available iron chelator used clinically for reduction of chronic iron overload in diseases such as β-thalassemia.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Acute 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML2673-BULK:
SML2673-VAR:
SML2673-50MG:
SML2673-10MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Kangna Cao et al.
European journal of clinical pharmacology, 76(1), 51-59 (2019-11-05)
Our aim was to evaluate the influence of genetic polymorphisms involved in the metabolism and transportation of deferasirox on deferasirox pharmacokinetics in the Chinese population. Thirty-eight healthy Chinese subjects were administered with a single dose of 20 mg kg-1 deferasirox.
Goldie Y L Lui et al.
Oncotarget, 6(22), 18748-18779 (2015-07-01)
Newer and more potent therapies are urgently needed to effectively treat advanced cancers that have developed resistance and metastasized. One such strategy is to target cancer cell iron metabolism, which is altered compared to normal cells and may facilitate their
Juan Daniel Díaz-García et al.
Nature reviews. Nephrology, 10(10), 574-586 (2014-07-23)
In 2005, the oral iron chelator deferasirox was approved by the FDA for clinical use as a first-line therapy for blood-transfusion-related iron overload. Nephrotoxicity is the most serious and frequent adverse effect of deferasirox treatment. This nephrotoxicity can present as
Claudia Bollig et al.
The Cochrane database of systematic reviews, 8, CD007476-CD007476 (2017-08-16)
Thalassaemia is a hereditary anaemia due to ineffective erythropoiesis. In particular, people with thalassaemia major develop secondary iron overload resulting from regular red blood cell transfusions. Iron chelation therapy is needed to prevent long-term complications.Both deferoxamine and deferiprone are effective;
Nesma Ahmed Safwat et al.
Pediatric research, 89(1), 185-190 (2020-06-17)
The genetic variants of the receptor for advanced glycation end products (RAGE) gene have been associated with vascular disease risk. The objective of this work was to explore the association of three single-nucleotide polymorphisms (SNPs) of RAGE gene (374T/A, 429T/C
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)