おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
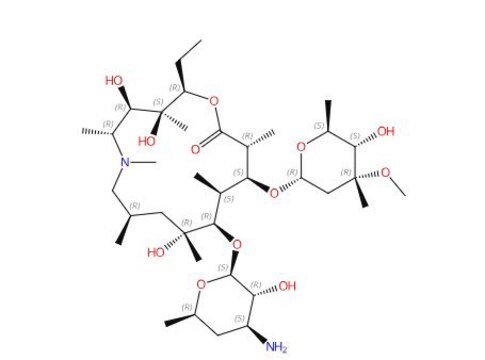
azithromycin
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
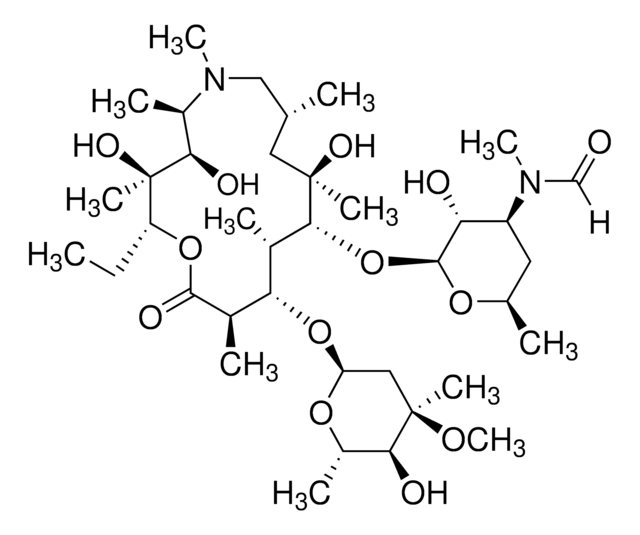
SMILES記法
N1([C@@H]([C@H]([C@@]([C@H](OC(=O)[C@@H]([C@H]([C@@H]([C@H]([C@@](C[C@H](C1)C)(O)C)O[C@@H]2O[C@@H](C[C@@H]([C@H]2O)N(C)C)C)C)O)C)CC)(O)C)O)C)C
InChI
1S/C30H58N2O9/c1-12-22-30(8,38)25(35)20(6)32(11)15-16(2)14-29(7,37)26(18(4)23(33)19(5)27(36)40-22)41-28-24(34)21(31(9)10)13-17(3)39-28/h16-26,28,33-35,37-38H,12-15H2,1-11H3/t16-,17-,18+,19-,20-,21+,22-,23+,24-,25-,26-,28+,29-,30-/m1/s1
InChI Key
PXDYILJJHOVNLO-NZMWSZMZSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
Desosaminylazithromycin USP reference standard intended for use in specified quality tests and assays.
Also used to prepare standard stock, system suitability, standard, system suitability stock, and peak identification solution for impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard stock, system suitability, standard, system suitability stock, and peak identification solution for impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
- Azithromycin for Injection
- Azithromycin
- Azithromycin Tablets
- Azithromycin for Oral Suspension
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Azithromycin for Injection
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 44(2), 451-451 (2020)
Azithromycin
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 43(3), 446-446 (2020)
Azithromycin for Oral Suspension
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 44(5) (2022)
Azithromycin Tablets
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 44(3), 457-457 (2020)
Fuad Al-Rimawi et al.
Journal of chromatographic science, 48(2), 86-90 (2010-01-30)
A simple, validated stability-indicating liquid chromatographic method is developed for the analysis of azithromycin in raw material and in pharmaceutical forms. Liquid chromatography with a UV detector at a wavelength of 210 nm using a reversed-phase C(18) stationary phase has
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)