1359415
USP
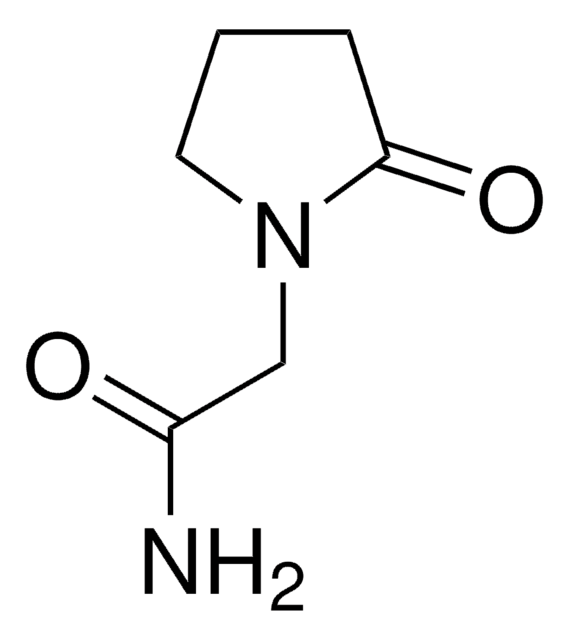
Levetiracetam Racemic Mixture
United States Pharmacopeia (USP) Reference Standard
別名:
(RS)-α-Ethyl-2-oxo-1-pyrrolidineacetamide, 2(RS)-(2-Oxopyrrolidin-1-yl)butyramide, Etiracetam
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C8H14N2O2
CAS番号:
分子量:
170.21
Beilstein:
1529106
MDL番号:
UNSPSCコード:
41116107
PubChem Substance ID:
NACRES:
NA.24
おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
levetiracetam
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
CCC(N1CCCC1=O)C(N)=O
InChI
1S/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)
InChI Key
HPHUVLMMVZITSG-UHFFFAOYSA-N
詳細
Levetiracetam racemic mixture also know as etiracetam is an stuctural analogue ethylated piracetam drug.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Levetiracetam Racemic Mixture USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Levetiracetam
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
1359415-15MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Aaron M Cook et al.
Neurocritical care, 19(2), 210-214 (2013-08-03)
Increased creatinine clearance and subsequent elevated antimicrobial clearance is evident in many traumatic brain injury (TBI) patients due to augmented renal clearance (ARC). Little is known about the effects of ARC on other renally-eliminated medications, such as the anti-epileptic drug
Can zinc depletion play a role in LEV-induced hair loss? Considerations from a case study.
Rocco Salvatore Calabrò et al.
Epilepsy & behavior : E&B, 29(1), 254-255 (2013-08-14)
S Shahbaz et al.
Dermatology online journal, 19(4), 4-4 (2013-09-12)
Drug Rash (or Reaction) with Eosinophilia and Systemic Symptoms (DRESS) is a potentially life-threatening hypersensitivity reaction to drugs characterized by rash, fever, lymphadenopathy, hematologic abnormalities, and involvement of internal organs. Initially coined in 1996, the term is used to refer
Young Jin Lee et al.
CNS drugs, 27(9), 753-759 (2013-08-08)
Antiepileptic drugs are commonly given for perioperative prophylaxis after brain tumor surgery, and there has been growing interest in levetiracetam, a second-generation antiepileptic drug. This retrospective study compared the seizure outcomes, side effects and durability of levetiracetam with valproic acid
Radica M Stepanovic-Petrovic et al.
Anesthesiology, 120(3), 737-750 (2013-09-28)
The β-lactam antibiotic ceftriaxone stimulates glutamate transporter GLT-1 expression and is effective in neuropathic and visceral pain models. This study examined the effects of ceftriaxone and its interactions with different analgesics (ibuprofen, celecoxib, paracetamol, and levetiracetam) in somatic and visceral
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)