おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
olanzapine
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
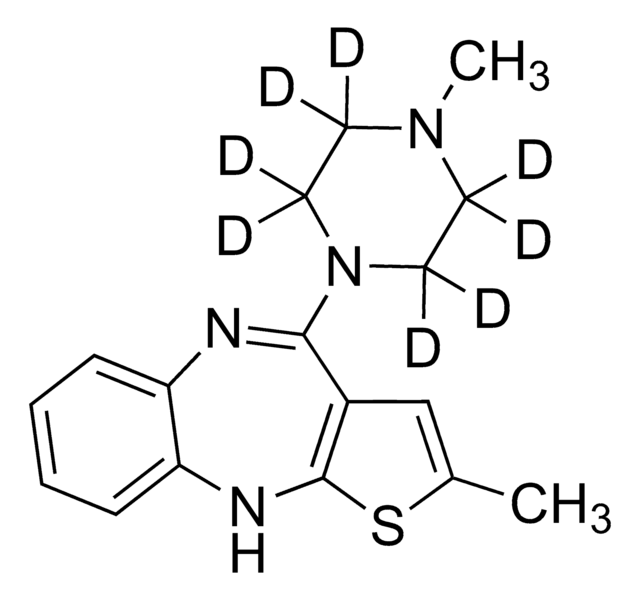
SMILES記法
CN1CCN(CC1)C2=Nc3ccccc3Nc4sc(C)cc24
InChI
1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
InChI Key
KVWDHTXUZHCGIO-UHFFFAOYSA-N
遺伝子情報
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356) , HTR2C(3358)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
- Olanzapine and Fluoxetine Capsules
- Olanzapine Orally Disintegrating Tablets
- Olanzapine Tablets
アナリシスノート
その他情報
関連製品
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
ターゲットの組織
Central nervous system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
1478301-200MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)

![5,10-ジヒドロ-2-メチル-4H--チエノ[2,3-b][1,5]ベンゾジアゼピン-4-オン Pharmaceutical Secondary Standard; Certified Reference Material](/deepweb/assets/sigmaaldrich/product/images/614/498/1e9cb612-e316-434a-b4af-7d6d53ef5794/640/1e9cb612-e316-434a-b4af-7d6d53ef5794.jpg)
![5-メチル-2-[(2-ニトロフェニル)アミノ]-3-チオフェンカルボニトリル Pharmaceutical Secondary Standard; Certified Reference Material](/deepweb/assets/sigmaaldrich/product/images/386/848/7a89608b-798f-44f2-ac8b-066ea654a260/640/7a89608b-798f-44f2-ac8b-066ea654a260.jpg)