111848
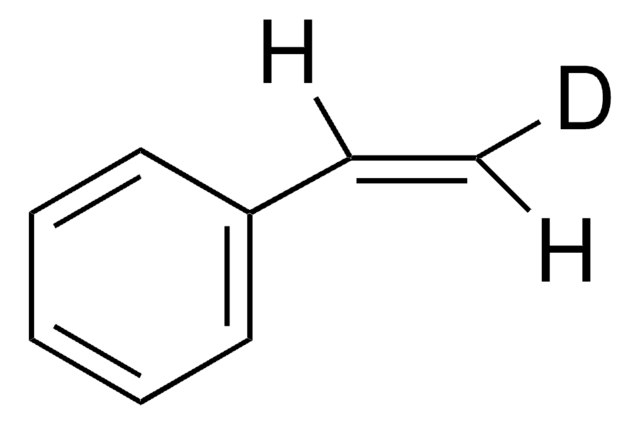
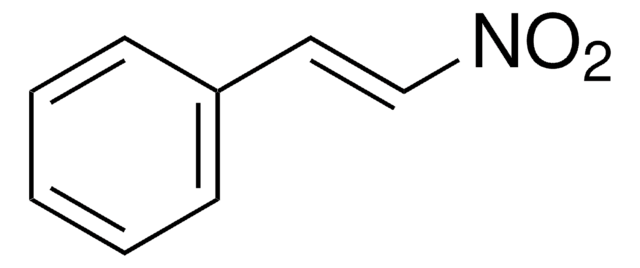
trans-β-Methylstyrene
99%
동의어(들):
trans-1-Phenyl-1-propene, trans-beta-Methylstyrene, trans-Propenylbenzene
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5CH=CHCH3
CAS Number:
Molecular Weight:
118.18
Beilstein:
1361672
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
포함
20 ppm 3,5-di-tert-butylcatechol as inhibitor
refractive index
n20/D 1.550 (lit.)
bp
175 °C (lit.)
density
0.911 g/mL at 25 °C (lit.)
작용기
phenyl
저장 온도
2-8°C
SMILES string
C\C=C\c1ccccc1
InChI
1S/C9H10/c1-2-6-9-7-4-3-5-8-9/h2-8H,1H3/b6-2+
InChI key
QROGIFZRVHSFLM-QHHAFSJGSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The product exhibits potential exothermic hazards at higher temperatures.
애플리케이션
Trans-β-methylstyrene was used in the epoxidation reaction of an oxidant solution with a solution of dichloromethane, where nickel is used as a catalyst and benzyltributylammonium bromide as a phase-transfer agent.
Trans-β-methylstyrene was used in the preparation of exo-chromans by the oxa analogue of the Povarov reaction.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 3 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
125.6 °F - closed cup
Flash Point (°C)
52 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
(Salen)nickel-Catalysed Epoxidations in the Homogeneous and Heterogeneous Phase: The Implications of Oxygen on the Efficiency and Product Selectivity.
Ferreira R, et al.
European Journal of Inorganic Chemistry, 2005(21), 4272-4279 (2005)
S Y Lin et al.
Journal of hazardous materials, 161(1), 330-335 (2008-05-20)
According to literature and our research, the styrene polymerization mechanism is identified by alpha-methylstyrene (AMS). This study investigated the basic exothermic behavior of styrene and its major derivatives, AMS, and trans-beta-methylstyrene (TBMS), by two calorimeters, differential scanning calorimetry (DSC) and
Rivka R R Taylor et al.
The Journal of organic chemistry, 78(4), 1404-1420 (2012-12-13)
An oxa analogue of the well-known Povarov reaction has been developed for the synthesis of 3,4-dihydrobenzopyrans (chromans). The reaction involves the formal inverse electron demand [4 + 2] cycloaddition reaction of in situ-generated cationic aryl 2-oxadiene oxocarbenium ions with alkenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.





![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)