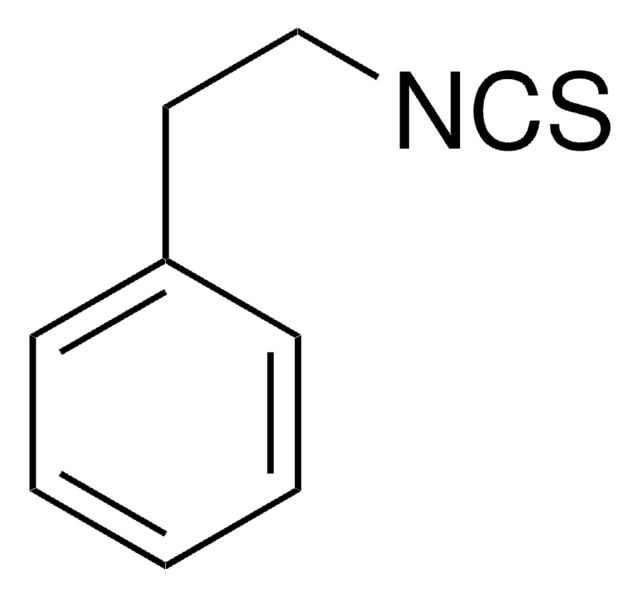
129453
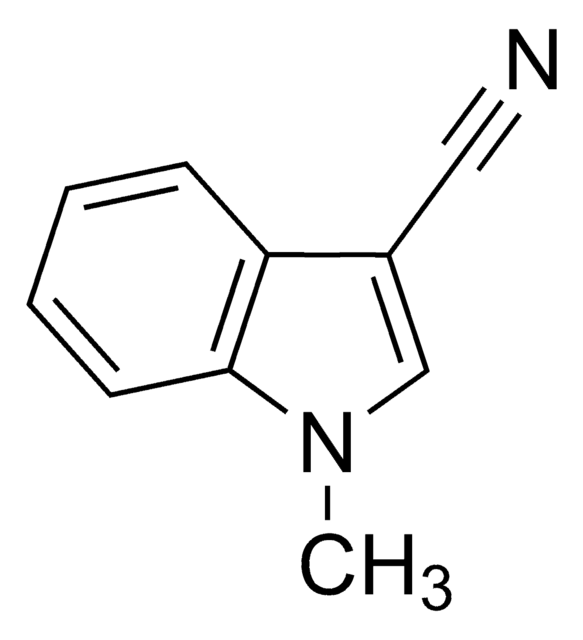
3-Indoleacetonitrile
98%
동의어(들):
(3-Indolyl)acetonitrile, 3-(Cyanomethyl)indole, IAN, Indolylacetonitrile, NSC 523272
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H8N2
CAS Number:
Molecular Weight:
156.18
Beilstein:
125488
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
solid
bp
157-160 °C/0.2 mmHg (lit.)
mp
33-36 °C (lit.)
작용기
nitrile
SMILES string
N#CCc1c[nH]c2ccccc12
InChI
1S/C10H8N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5H2
InChI key
DMCPFOBLJMLSNX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Indoleacetonitrile (Indolylacetonitrile) is a light-induced auxin-inhibitory substance that is isolated from light-grown cabbage (Brassica olearea L.) shoots. It inhibits the biofilm formation of both E. coli O157:H7 and P. aeruginosa without affecting its growth.
애플리케이션
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Histone deacetylase inhibitors
- Potential kinase inhibitors
- Kv7/KCNQ potassium channel activators
- Kinesin-Specific MKLP-2 Inhibitor
- Pesticides
- Potential PET cancer imaging agents
- Agonists of the Farnesoid X Receptor (FXR) as atherosclerosis treatment
- Butyrylcholinesterase inhibitors
- Necroptosis inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
233.6 °F - closed cup
Flash Point (°C)
112 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Light-induced auxin-inhibiting substance from cabbage (Brassica oleacea L.) shoots.
Kosemura S, et al.
Tetrahedron Letters, 38(48), 8327-8330 (1997)
Jin-Hyung Lee et al.
Environmental microbiology, 13(1), 62-73 (2010-07-24)
Intercellular signal indole and its derivative hydroxyindoles inhibit Escherichia coli biofilm and diminish Pseudomonas aeruginosa virulence. However, indole and bacterial indole derivatives are unstable in the microbial community because they are quickly degraded by diverse bacterial oxygenases. Hence, this work
Tongbing Su et al.
The Plant cell, 23(1), 364-380 (2011-01-18)
Camalexin, a major phytoalexin in Arabidopsis thaliana, consists of an indole ring and a thiazole ring. The indole ring is produced from Trp, which is converted to indole-3-acetonitrile (IAN) by CYP79B2/CYP79B3 and CYP71A13. Conversion of Cys(IAN) to dihydrocamalexic acid and
J Normanly et al.
The Plant cell, 9(10), 1781-1790 (1997-11-22)
Indole-3-acetonitrile (IAN) is a candidate precursor of the plant growth hormone indole-3-acetic acid (IAA). We demonstrated that IAN has auxinlike effects on Arabidopsis seedlings and that exogenous IAN is converted to IAA in vivo. We isolated mutants with reduced sensitivity
Satoko Sugawara et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5430-5435 (2009-03-13)
Auxins are hormones that regulate many aspects of plant growth and development. The main plant auxin is indole-3-acetic acid (IAA), whose biosynthetic pathway is not fully understood. Indole-3-acetaldoxime (IAOx) has been proposed to be a key intermediate in the synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.