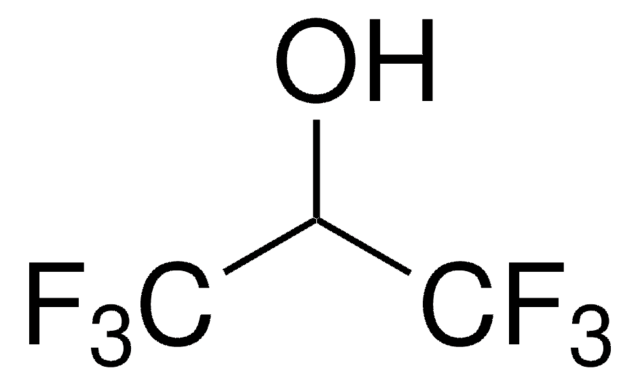
추천 제품
일반 설명
cis-2-phenyl-1,3-dioxan-5-ol reacts with triphenylphosphine-carbon tetrabromide to yield cis-4-bromomethyl-2-phenyl-1,3-dioxolan, its trans-diastereoisomer and trans-5-bromo-2-phenyl-1,3-dioxan.
애플리케이션
2-Phenyl-1,3-dioxan-5-ol can be used as a starting material in the synthesis of 5-benzyloxy1,3-dioxan-2-one (a carbonate of glycerol monomer). It undergoes ring-opening polymerization with ε-caprolactone in presence of tin(II) 2-ethylhexanoate (Sn(oct)2).
2-Phenyl-1,3-dioxan-5-ol was used as starting reagent in the synthesis of carbonate of glycerol monomer, 5-benzyloxy-1,3-dioxan-2-one which forms copolymers with ε-caprolactone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Poly (carbonate ester) s based on units of 6-hydroxyhexanoic acid and glycerol.
Wolinsky JB, et al.
Macromolecules, 40(20), 7065-7068 (2007)
Nucleophilic substitution in glycerol derivatives. Part VI. Halogenodeoxygenation of 2-phenyl-1, 3-dioxan-5-ol to give 1, 3-dioxans and 1, 3-dioxolans.
Aneja R and Davies AP.
Journal of the Chemical Society. Perkin Transactions 1, 141-145 (1974)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.