추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
mp
102-105 °C (lit.)
solubility
ethanol: soluble 100 mg/mL, clear, faintly yellow
작용기
carboxylic acid
chloro
SMILES string
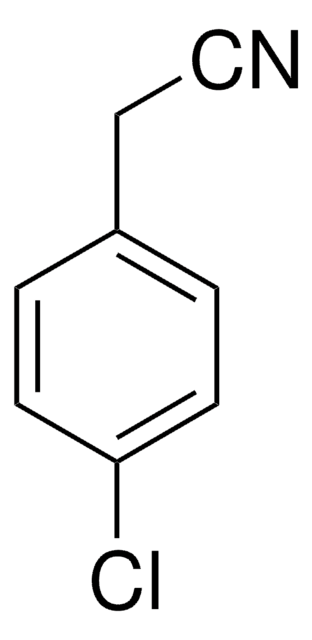
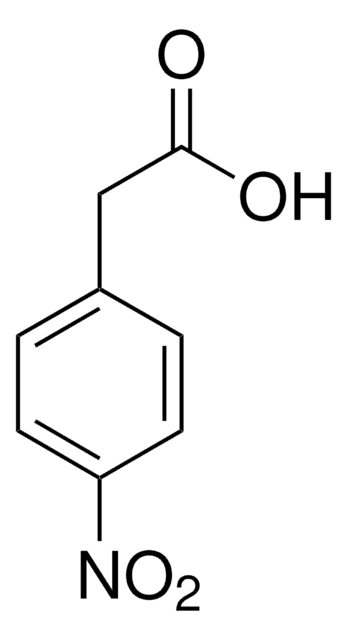
OC(=O)Cc1ccc(Cl)cc1
InChI
1S/C8H7ClO2/c9-7-3-1-6(2-4-7)5-8(10)11/h1-4H,5H2,(H,10,11)
InChI key
CDPKJZJVTHSESZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Chlorophenylacetic acid possess anticancer properties. It is a novel therapeutic agent which can be useful in prevention or treatment of estrogen-sensitive breast cancer. It acts as carbon and energy supplement and is degraded by Pseudomonas sp. strain CBS3.
애플리케이션
4-Chlorophenylacetic acid was used to study the mechanism of aerobic degradation of 1,1-dichloro-2,2-bis(4-chlorophenyl)ethane by Ralstonia eutropha A5.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Hay et al.
FEMS microbiology ecology, 31(3), 249-253 (2000-03-17)
Evidence is presented demonstrating the ability of Ralstonia eutropha A5 to degrade 1,1-dichloro-2,2-bis(4-chlorophenyl)ethane (DDD) aerobically. Strain A5 was able to effect significant transformation of [(14)C]DDD: the hexane extractable radioactivity decreased to approximately 50% of the controls while more than 25%
S Sawatsri et al.
International journal of cancer, 93(5), 687-692 (2001-07-31)
The aromatic fatty acid phenylacetate (PA) and its analogs have come under intense investigation due to their ability to cause the growth arrest of a variety of neoplasia, including human breast cancer. We have determined that PA and its halide
N Sidell et al.
British journal of cancer, 89(2), 412-419 (2003-07-17)
We have investigated the effects of the low-toxic retinoid, all-trans retinoyl beta-glucuronide (RAG) alone and in combination with the phenylacetate (PA) derivative 4-chloro-phenylacetate (4-CPA) on the human neuroblastoma cell line, LA-N-5. In vitro studies demonstrated that RAG and 4-CPA treatments
Neil Sidell et al.
Cancer letters, 251(2), 302-310 (2007-01-12)
Treatment of estrogen-sensitive breast cancer with selective estrogen selective modulators (SERMs) and, more recently, aromatase inhibitors has met with wide success. However, antagonism of estrogen receptor (ER) activity in breast carcinomas by SERMs such as tamoxifen has been associated with
A Markus et al.
Journal of bacteriology, 160(2), 618-621 (1984-11-01)
In cell-free extracts from Pseudomonas sp. strain CBS3 the conversion of 4-chlorophenylacetate to 3,4-dihydroxyphenylacetate was demonstrated. By Sephacryl S-200 chromatography two protein fractions, A and B, were obtained which both were essential for enzyme activity. Fe2+ and NADH were cofactors
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.