추천 제품
일반 설명
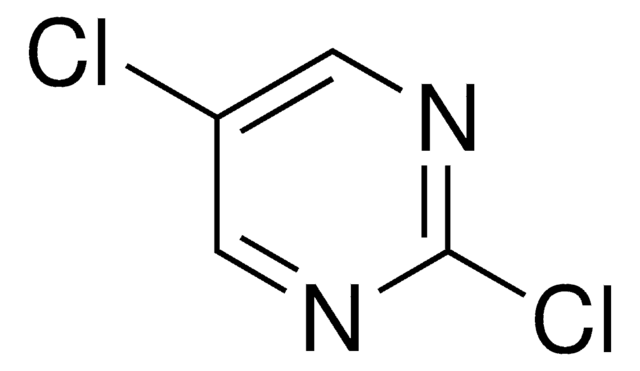
Cyclic voltammograms of 4,6-dichloropyrimidine shows three cathodic waves, arising from sequential cleavage of carbon-chlorine bonds as well as the reduction of pyrimidine.
애플리케이션
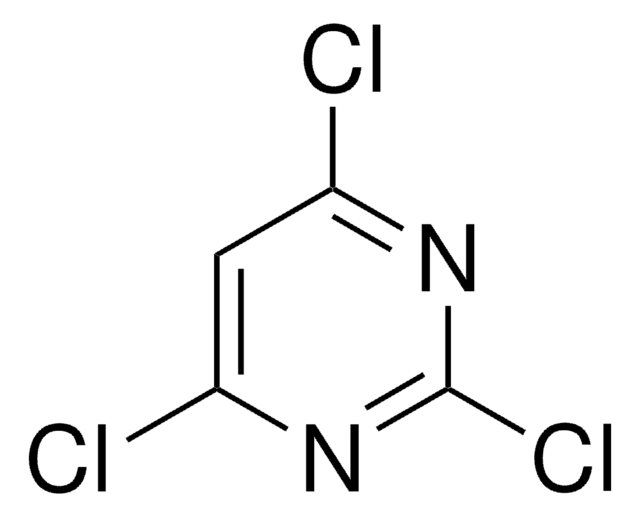
4,6-Dichloropyrimidine was used in the synthesis of N-substituted azacalix[4]pyrimidines. It was used as starting reagent for the synthesis of disubstituted pyrimidines by tandem amination and Suzuki-Miyaura cross-coupling. It was also used in a biarylpyrimidine synthesis involving biaryl cross-coupling.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Richard T Wheelhouse et al.
Journal of medicinal chemistry, 49(17), 5187-5198 (2006-08-18)
Biarylpyrimidines are characterized as selective ligands for higher-order nucleic acid structures. A concise and efficient synthesis has been devised incorporating Suzuki biaryl cross-coupling of dihalopyrimidines. Two ligand series are described based on the parent thioether 4,6-bis[4-[[2-(dimethylamino)ethyl]mercapto]phenyl]pyrimidine (1a) and amide 4,6-bis(4[(2-(dimethylamino)ethyl)carboxamido]phenyl)pyrimidine
Li-Xia Wang et al.
The Journal of organic chemistry, 75(3), 741-747 (2010-01-02)
A number of N-substituted azacalix[4]pyrimidines were synthesized by two methods. While straightforward condensation reaction between 4,6-dichloropyrimidine and 4,6-bis(alkylamino)pyrimidines gave identically N-substituted azacalix[4]pyrimidines in low yields, a general and moderate-to-high yielding 1 + 3 macrocyclic fragment coupling reaction afforded azacalix[4]pyrimidines that
Tetrahedron, 62, 10055-10055 (2006)
Electrochemical reduction of halogenated pyrimidines at mercury cathodes in acetonitrile.
Ji C, et al.
Journal of Electroanalytical Chemistry, 500(1), 3-11 (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.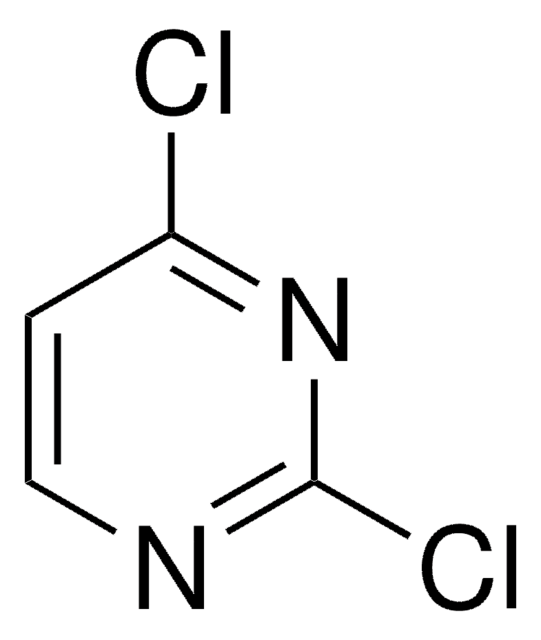



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)