추천 제품
vapor density
>1 (vs air)
Quality Level
vapor pressure
<0.01 mmHg ( 20 °C)
분석
98%
양식
liquid
refractive index
n20/D 1.578 (lit.)
bp
278-282 °C/760 mmHg (lit.)
density
1.094 g/mL at 25 °C (lit.)
SMILES string
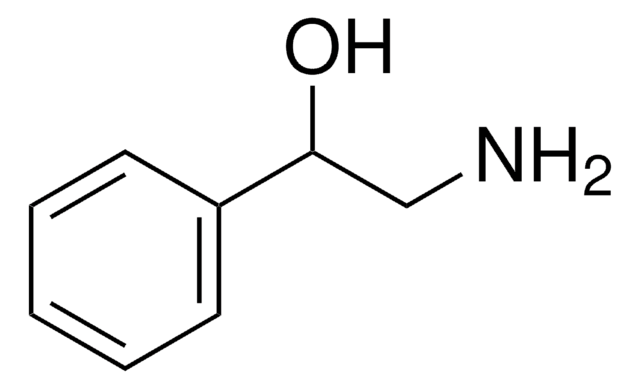
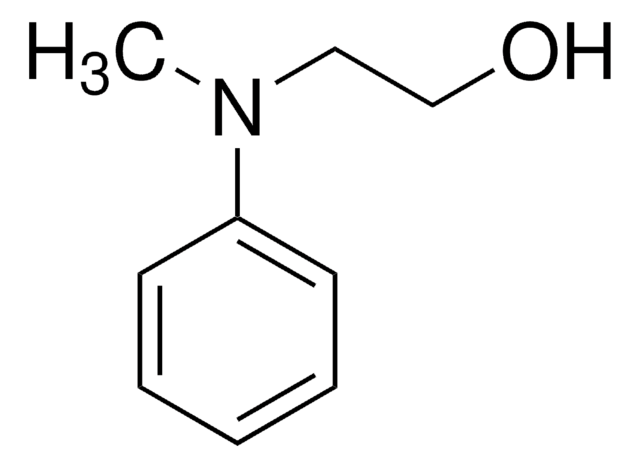
OCCNc1ccccc1
InChI
1S/C8H11NO/c10-7-6-9-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2
InChI key
MWGATWIBSKHFMR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
N-(2-Hydroxyethyl)aniline was employed as substrate for human olfactory UDP-glucuronosyltransferase.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
표적 기관
Blood, Blood,hematopoietic system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Mercedes Amat et al.
Natural product communications, 6(4), 515-526 (2011-05-13)
This review is focused on recent synthetic achievements and ongoing work in our laboratory using phenylglycinol-derived oxazolopiperidone lactams as starting materials for the enantioselective synthesis of piperidine-containing alkaloids: madangamines, 2,5-disubstituted decahydroquinoline and 1-substituted tetrahydroisoquinoline alkaloids, the indole alkaloids 20S- and
M Amat et al.
Organic letters, 3(21), 3257-3260 (2001-10-12)
[reaction: see text]. The phenylglycinol-derived 2-pyridone 1 undergoes m-CPBA oxidation steroselectively leading to the chiral nonracemic unsaturated bicyclic hydroxylactam 2, from which the enantioselective synthesis of (3R,5R)-3,4,5-trihydroxypiperidine (16) and the formal synthesis of the azasugar epiisofagomine are described. The enantioselective
Mercedes Amat et al.
Organic & biomolecular chemistry, 9(7), 2175-2184 (2011-02-08)
The double cyclocondensation of symmetric pyridyl bis(oxoacids) 2b and 3b with (R)-phenylglycinol stereoselectively gave access to bis-phenylglycinol-derived oxazolopyrrolidine 9 and oxazolopiperidone 10, respectively. Application of the stereocontrolled cyclocondensation reaction to phenyl bis-γ-oxoacid 4b provided 11, which was converted to the
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7724-7732 (2011-06-15)
Phenylglycinol-derived, unsaturated oxazolopiperidone lactams are extremely useful building blocks that undergo stereoselective conjugate addition reactions with organocuprates, enolates, and sulfur-stabilized anions, allowing the stereocontrolled introduction of substituents at the piperidine 4-position. The factors governing the exo- or endo-facial selectivity are
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(30), 7872-7881 (2006-07-20)
A straightforward procedure for the synthesis of enantiopure polysubstituted piperidines is reported. It involves the direct generation of chiral non-racemic oxazolo[3,2-a]piperidone lactams that already incorporate carbon substituents on the heterocyclic ring and the subsequent removal of the chiral auxiliary. The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.