158496
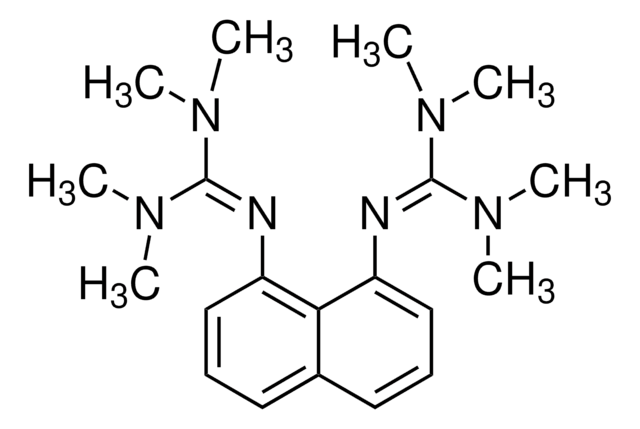
Proton-sponge®
99%
동의어(들):
1,8-Bis(dimethylamino)naphthalene, N,N,N′,N′-Tetramethyl-1,8-naphthalenediamine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C10H6[N(CH3)2]2
CAS Number:
Molecular Weight:
214.31
Beilstein:
396782
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
solid
mp
49-51 °C (lit.)
solubility
chloroform: soluble 50 mg/mL, clear (faint yellow to dark yellow to dark red)
작용기
amine
SMILES string
CN(C)c1cccc2cccc(N(C)C)c12
InChI
1S/C14H18N2/c1-15(2)12-9-5-7-11-8-6-10-13(14(11)12)16(3)4/h5-10H,1-4H3
InChI key
GJFNRSDCSTVPCJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Proton-sponge is a nucleophilic base used in alkoxycarbonylation, dehydrofluorination, arylation, and intramolecular cyclization reactions.
Proton-sponge is also referred to as 1,8-dimethylamino naphthalene. It is very strong base with weak nucleophilic character due to steric effects. It also participates in the reactions between arachno-6,9-C2B8H14 and selected acyl chlorides. It has been tested as an effective H+ scavenger.
Proton-sponge is also referred to as 1,8-dimethylamino naphthalene. It is very strong base with weak nucleophilic character due to steric effects. It also participates in the reactions between arachno-6,9-C2B8H14 and selected acyl chlorides. It has been tested as an effective H+ scavenger.
애플리케이션
Proton-sponge can be used in the synthesis of C-substituted t-BuNH-8,9-R,R′-nido-7,8,9-C3B8H9 (R,R′ = H,H; MeH; Me,Me; Ph,H and Ph,Ph) tricarbollide compounds. It was also used in the preparation of saturated fluoroalkyl(hydrido) complexes of Iridium.
Very strong base with weak nucleophilic character due to steric effects.
법적 정보
Proton-sponge is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Unusual Reactivity of ?Proton Sponge? as a Hydride Donor to Transition Metals: Synthesis and Structural Characterization of Fluoroalkyl (hydrido) Complexes of Iridium (III) and Rhodium (III).
Hughes RP, et al.
Organometallics, 20(14), 3190-3197 (2014)
Nanocluster Formation and Stabilization Fundamental Studies. 2. Proton Sponge as an Effective H^+ Scavenger and Expansion of the Anion Stabilization Ability Series.
Ozkar S and Finke RG.
Langmuir, 18(20), 7653-7662 (2002)
J. Chem. Soc. Perkin Trans. II, 857-857 (1991)
Mario Bakardjiev et al.
Dalton transactions (Cambridge, England : 2003), 39(17), 4186-4190 (2010-04-15)
Treatment of C-substituted nido dicarbadecaboranes 5,6-R',R-5,6-C(2)B(8)H(10) (1) (where R',R = H,H (1a); H,Me, (1b); Me,Me, (1c); H,Ph, (1d) and Ph,Ph, (1e) with 1,8-bis-(dimethylamino)naphthalene (proton sponge = PS) and t-BuNC in CH(2)Cl(2), followed by acidification, generated a series of pure neutral
1, 8-Bis (dimethylamino) naphthalene
Barner BA
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)
![2,8,9-Triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo[3,3,3]undecane](/deepweb/assets/sigmaaldrich/product/structures/387/021/edaffe12-6e4b-4305-9030-749551ac828a/640/edaffe12-6e4b-4305-9030-749551ac828a.png)

![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)