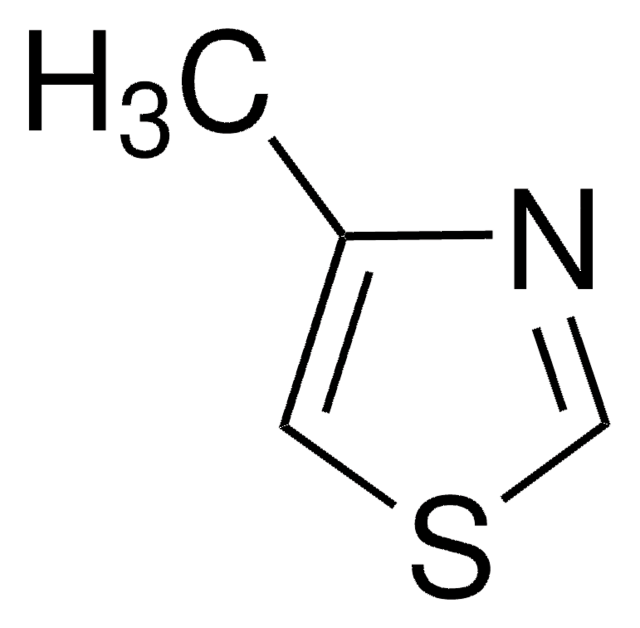
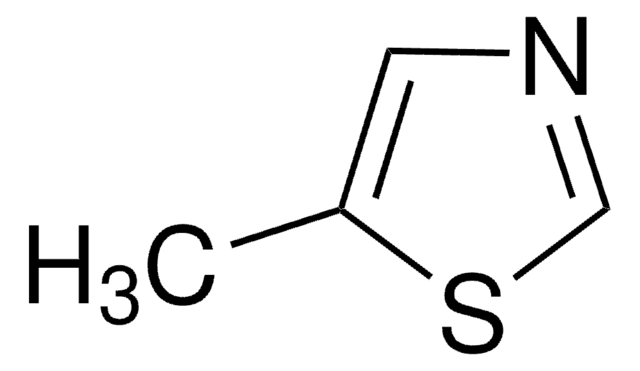
추천 제품
애플리케이션
2-Bromothiazole was used to N-arylate 5- and 7-azaindoles. 2-Bromothiazole was also used as starting reagent in the synthesis of:
- 2-cyanothiazole via cpper-catalyzed cyanation
- 2,4,5-trisubstituted thiazoles
- novel electron-deficient fused pyrrolo[3,2-d:4,5-d′]bisthiazole
- 3-(2′-thiazoyl)indoles
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point (°F)
closed cup - does not flash
Flash Point (°C)
closed cup - does not flash
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Synlett, 555-555 (2007)
Tetrahedron Letters, 48, 4831-4831 (2007)
Mohammed Al-Hashimi et al.
Organic letters, 12(23), 5478-5481 (2010-11-12)
The synthesis of a novel electron-deficient fused pyrrolo[3,2-d:4,5-d']bisthiazole is reported from 2-bromothiazole. This was copolymerized with thiophene, selenophene, thienothiophene, and bithiophene by microwave-assisted Stille polycondensation. The resulting polymers exhibited small optical band gaps combined with low-lying HOMO energy levels and
Synthesis of camalexin and related phytoalexins.
Ayer WA, et al.
Tetrahedron, 48(14), 2919-2924 (1992)
Cora Dunst et al.
The Journal of organic chemistry, 76(16), 6972-6978 (2011-07-09)
A general method for the synthesis of 2,4,5-trisubstituted thiazoles has been developed. Starting from commercially available 2-bromothiazole, successive metalations using TMPMgCl·LiCl or TMP(2)Zn·2MgCl(2)·2LiCl lead to the corresponding magnesated or zincated thiazoles which readily react with various electrophiles providing highly functionalized
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)