162698
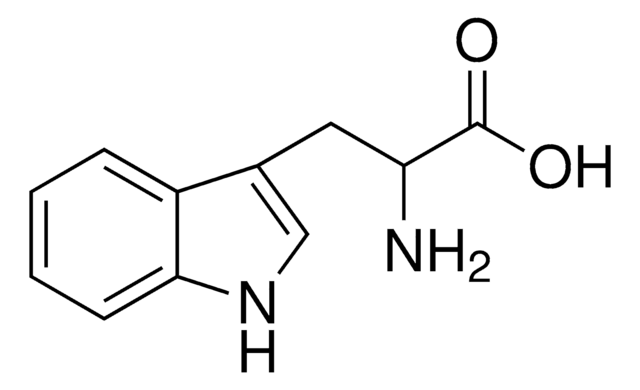
DL-Tryptophan
≥99% (HPLC), for peptide synthesis
동의어(들):
(±)-α-Amino-3-indolepropionic acid, (±)-2-Amino-3-(3-indolyl)propionic acid, DL-3β-Indolylalanine
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C11H12N2O2
CAS Number:
Molecular Weight:
204.23
Beilstein:
86199
EC Number:
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.22
추천 제품
제품명
DL-Tryptophan, ≥99% (HPLC)
Quality Level
분석
≥99% (HPLC)
양식
powder
반응 적합성
reaction type: solution phase peptide synthesis
mp
289-290 °C (dec.) (lit.)
응용 분야
peptide synthesis
SMILES string
NC(Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)
InChI key
QIVBCDIJIAJPQS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
DL-Tryptophan also known as 2-amino-3-(1H-indol-3-yl)-propionic acid, is commonly used in peptide synthesis.
애플리케이션
DL-Tryptophan is used as a starting material for the preparation of N-acyl monoisotripeptides via solution-phase peptide synthesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Solution-phase synthesis of chiral N-, O-, and S-acyl isopeptides
S Liaqat
Synthesis, 46, 67-72 (2014)
Aristeidis Chiotellis et al.
Molecular pharmaceutics, 11(11), 3839-3851 (2014-07-06)
As a continuation of our research efforts toward the development of tryptophan-based radiotracers for tumor imaging with positron emission tomography (PET), three new fluoroethoxy tryptophan analogues were synthesized and evaluated in vivo. These new tracers (namely, 4-(2-[(18)F]fluoroethoxy)-dl-tryptophan ([(18)F]4-FEHTP), 6-(2-[(18)F]fluoroethoxy)-dl-tryptophan ([(18)F]6-FEHTP)
Helen Ling et al.
Movement disorders : official journal of the Movement Disorder Society, 30(7), 960-967 (2015-04-10)
Glial cytoplasmic inclusions containing α-synuclein are the pathological hallmark of multiple system atrophy (MSA). Minimal change (MC-MSA) is an unusual MSA subtype with neuronal loss largely restricted to the substantia nigra and locus coeruleus. Immunohistochemistry on selected brain regions and
Mariano Soiza-Reilly et al.
Neuropharmacology, 89, 185-192 (2014-09-28)
5-HT1A receptors are widely expressed in the brain and play a critical role in feedback inhibition of serotonin (5-HT) neurons through multiple mechanisms. Yet, it remains poorly understood how these feedback mechanisms, particularly those involving long-range projections, adapt in mood
Kazuo Tatebayashi et al.
Nature communications, 6, 6975-6975 (2015-04-22)
The yeast high osmolarity glycerol (HOG) pathway activates the Hog1 MAP kinase, which coordinates adaptation to high osmolarity conditions. Here we demonstrate that the four-transmembrane (TM) domain protein Sho1 is an osmosensor in the HKR1 sub-branch of the HOG pathway.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.