모든 사진(2)
About This Item
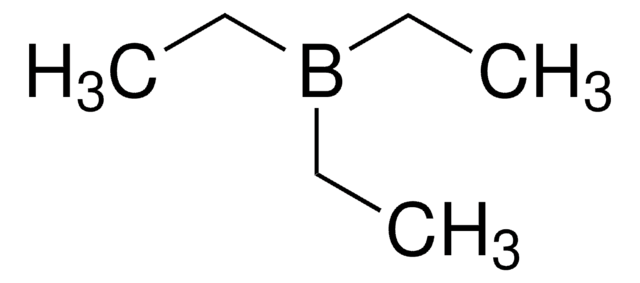
Linear Formula:
(C2H5)3B
CAS Number:
Molecular Weight:
97.99
Beilstein:
1731462
MDL number:
UNSPSC 코드:
12352001
eCl@ss:
38120201
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Catalyst for:
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
Triethylborane is used as a catalyst for:
It can be employed as a reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes and for the synthesis of tetramethylammonium trialkylphenylborate salts.
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
It can be employed as a reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes and for the synthesis of tetramethylammonium trialkylphenylborate salts.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1A - STOT SE 3 - Water-react 2
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Highly enantioselective palladium-catalyzed umpolung allylation of aldehydes.
Zhu, Shou-Fei and Qiao, Xiang-Chen and Zhang, Yong-Zhen and Wang, Li-Xin and Zhou, Qi-Lin
Chemical Science, 2(6), 1135-1140 (2011)
Rhenium hydride/boron Lewis acid cocatalysis of alkene hydrogenations: Activities comparable to those of precious metal systems.
Jiang, Yanfeng and Hess, Jeannine and Fox, Thomas and Berke, Heinz
Journal of the American Chemical Society, 132(51), 18233-18247 (2010)
Formation of Ketenimines via the Palladium-Catalyzed Decarboxylative π-Allylic Rearrangement of N-Alloc Ynamides.
Alexander, Juliana R and Cook, Matthew J
Organic Letters, 19(21), 5822-5825 (2017)
Aerobic Hydroxylation of N-Borylenamine: Triethylborane-Mediated Hydroxyalkylation of α, β-Unsaturated Oxime Ether.
Ueda M, et al.
Organic Letters, 11(20), 4632-4635 (2009)
Synthesis of N-heterocyclic carbene boranes via silver N-heterocyclic carbene complexes.
Ono S, et al.
Polyhedron, 137(20), 296-305 (2017)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.