추천 제품
일반 설명
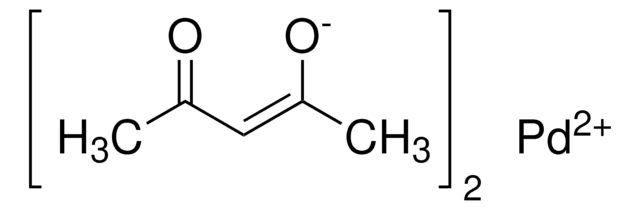
Palladium(II) acetylacetonate (Pd(acac)2) is a metal-organic complex. Sublimation of Pd(acac)2 has been investigated by thermogravimetry and XRD. The temperature range for the sublimation of Pd(acac)2, without undergoing thermal decomposition, was determined to be 100-160°C in the presence of inert gas helium.
애플리케이션
Palladium(II) acetylacetonate (Pd(acac)2) was used in the following studies:
- Typical high-temperature organic solution phase protocol for the preparation of monodisperse CuPd alloy nanoparticles (NPs).
- Preparation of [(NHC)Pd(acac)L] (where L=Me, NHC = N-heterocyclic carbene) complexes. These complexes efficiently catalyze the Heck reaction of activated aryl bromides.
- As catalyst in the decarboxylative cross-coupling of arylcarboxylic acids with aryl halides.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Sublimation and deposition behaviour of palladium (II) acetylacetonate.
Cominos V and Gavriilidis A.
The European Physical Journal - Applied Physics, 15(01), 23-33 (2001)
Lukas J Goossen et al.
Journal of the American Chemical Society, 129(15), 4824-4833 (2007-03-23)
A new strategy for the regiospecific construction of unsymmetrical biaryls is presented, in which easily available salts of carboxylic acids are decarboxylated in situ to give arylmetal species that serve as the nucleophilic component in a catalytic cross-coupling reaction with
Nicolas Marion et al.
Accounts of chemical research, 41(11), 1440-1449 (2008-09-09)
Metal-catalyzed cross-coupling reactions, notably those permitting C-C bond formation, have witnessed a meteoritic development and are now routinely employed as a powerful synthetic tool both in academia and in industry. In this context, palladium is arguably the most studied transition
Sümeyra Diyarbakir et al.
ACS applied materials & interfaces, 7(5), 3199-3206 (2015-01-17)
Monodisperse CuPd alloy nanoparticles (NPs) were prepared by using a typical high-temperature organic solution phase protocol comprising the coreduction of copper(II) acetylacetonate and palladium(II) acetylacetonate by morpholine-borane complex in oleylamine and 1-octadecene solution at 80 °C. The presented synthesis protocol
Paul Chatelain et al.
Angewandte Chemie (International ed. in English), 58(42), 14959-14963 (2019-08-24)
Ideal organic syntheses involve the rapid construction of C-C bonds, with minimal use of functional group interconversions. The Suzuki-Miyaura cross-coupling (SMC) is a powerful way to form biaryl linkages, but the relatively similar reactivity of electrophilic partners makes iterative syntheses
문서
High Purity Metalorganic Precursors for CPV Device Fabrication
The properties of many devices are limited by the intrinsic properties of the materials that compose them.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.