추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.552 (lit.)
bp
110-111 °C/14 mmHg (lit.)
density
1.144 g/mL at 25 °C (lit.)
작용기
hydroxyl
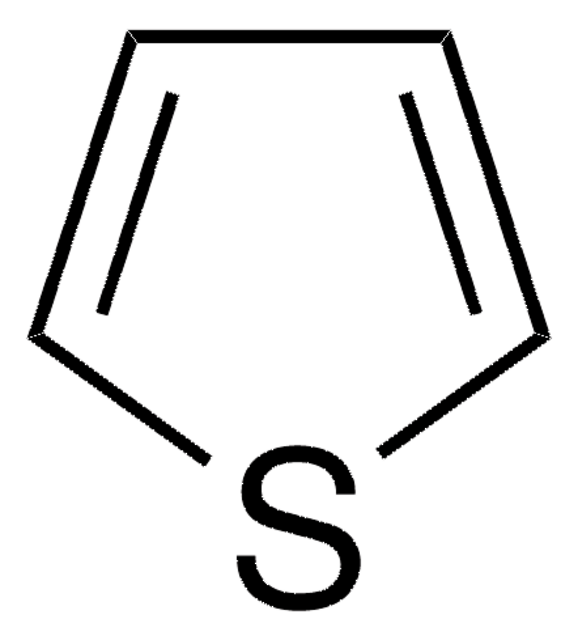
SMILES string
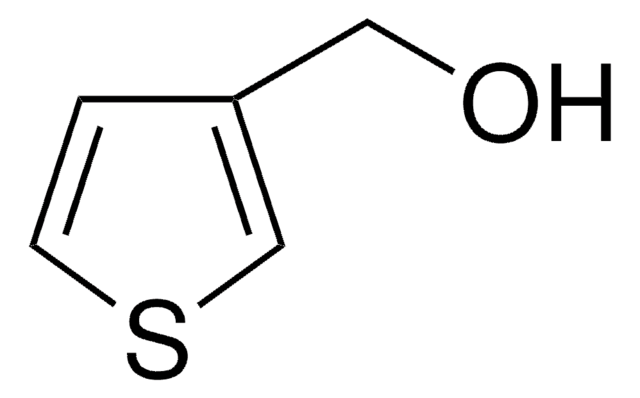
OCCc1ccsc1
InChI
1S/C6H8OS/c7-3-1-6-2-4-8-5-6/h2,4-5,7H,1,3H2
InChI key
YYPNNBPPDFTQFX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Thiopheneethanol [3-(2-hydroxyethyl)thiophene] was used in the synthesis of various ether and ester derivatives such as 3-(2-(benzyloxy)ethyl)thiophene, 3-[2-((triphenylmethyl)oxy)ethyl]thiophene, 3-[2-((trimethylsilyl)oxy)ethyl]thiophene, 3-[2-((dimethyl-tert-butylsilyl)oxy)ethyl]thiophene, 3-(2-acetoxyethyl)thiophene and 3-(2-(benzoyloxy)ethyl)thiophene.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
197.6 °F - closed cup
Flash Point (°C)
92 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Reactive groups on polymer-covered electrodes. 5. Synthesis and cyclovoltammetric analysis of 3-substituted thiophene derivatives.
Macromolecules, 30(24), 7419-7426 (1997)
Consuelo Ripoll et al.
Pharmaceutics, 13(2) (2021-03-07)
Recently, it was proposed that the thiophene ring is capable of promoting mitochondrial accumulation when linked to fluorescent markers. As a noncharged group, thiophene presents several advantages from a synthetic point of view, making it easier to incorporate such a
Yuwei Hao et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 19(16), 2046-2051 (2018-03-25)
Highly efficient cell capture and release with low background are urgently required for early diagnosis of diseases such as cancer. Herein, we report an electrochemical responsive superhydrophilic surface exhibiting specific cell capture and release with high yields and extremely low
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)