241814
Lithium tri-tert-butoxyaluminum hydride solution
1.0 M in THF
동의어(들):
LTTBA, Lithium hydrotri-tert-butoxyaluminate, Tri-tert-butoxyaluminumlithium hydride
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
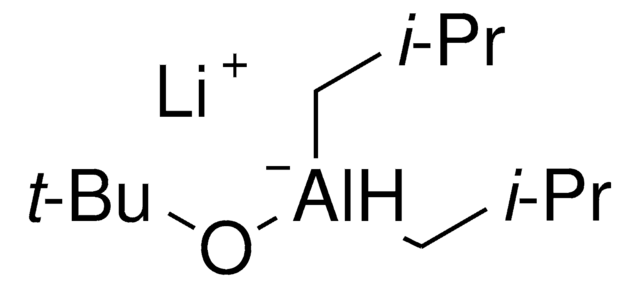
LiAlH[OC(CH3)3]3
CAS Number:
Molecular Weight:
254.27
Beilstein:
5796791
MDL number:
UNSPSC 코드:
12352000
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
1.0 M in THF
density
0.904 g/mL at 25 °C
SMILES string
[Li+].CC(C)(C)O[AlH-](OC(C)(C)C)OC(C)(C)C
InChI
1S/3C4H9O.Al.Li.H/c3*1-4(2,3)5;;;/h3*1-3H3;;;/q3*-1;+2;+1;
InChI key
BYBIDFZFKATBFH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lithium tri-tert-butoxyaluminum hydride is a mild reducing agent mainly used for the selective reduction of ketones.
애플리케이션
- Reducing agent
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis of an Anti???Amino Epoxide by One?Carbon Homologation of an ??Amino Ester:(2S, 3S)?1, 2?Epoxy?3?(Boc?Amino)?4?Phenylbutane.
Wang D & Nugent WA
Organic Syntheses, 58-67 (2007)
The use of 4, 4-disubstituted nopinones for natural-product synthesis. Synthesis of elemanoid sesquiterpenes.
Kato M, et al.
The Journal of Organic Chemistry, 56(25), 7071-7076 (1991)
Stereoselectivity of lithium tri-tert-butoxyaluminum hydride.
Ashby EC, et al.
The Journal of Organic Chemistry, 36(1), 197-199 (1971)
Synthesis of an enantiomerically pure intermediate containing the CD substructure of taxol.
Isaacs RC, et al.
The Journal of Organic Chemistry, 58(15), 3938-3941 (1993)
M Gobbini et al.
Bioorganic & medicinal chemistry, 6(10), 1889-1894 (1998-12-05)
The four stereoisomers of the 2-hydroxy derivatives of digitoxigenin and 3-epidigitoxigenin have been synthesized, their structures established by NMR, and their binding affinity for the digitalis receptor on Na+, K(+)-ATPase evaluated. These derivatives showed lower affinities than the parent compounds.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.