289094
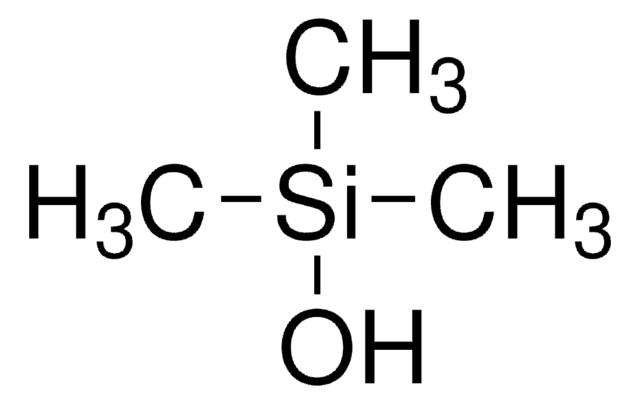
tert-Butyldimethylsilanol
99%
동의어(들):
1-(1,1-Dimethylethyl)-1,1-dimethylsilanol, tert-Butyl(hydroxy)dimethylsilane, tert-Butyldimethylsilanol, tert-Butyldimethylsilyl hydroxide, Dimethyl-tert-butylsilanol
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
(CH3)3CSi(CH3)2OH
CAS Number:
Molecular Weight:
132.28
Beilstein:
1732777
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.424 (lit.)
bp
139 °C/739 mmHg (lit.)
density
0.84 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)O
InChI
1S/C6H16OSi/c1-6(2,3)8(4,5)7/h7H,1-5H3
InChI key
FGWRMMTYIZKYMA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
tert-Butyldimethylsilanol can be used as a silylating agent for the protection of hydroxyl groups via silylation. It is used:
- For the preparation of α-chiral ether derivatives by catalytic asymmetric allylic substitution.
- As an initiator for the polymerization of 1,2 benzenedicarboxaldehyde.
- In the synthesis of enol silyl ethers.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
113.0 °F - closed cup
Flash Point (°C)
45 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Reproducible and scalable synthesis of end-cap-functionalized depolymerizable poly (phthalaldehydes)
DiLauro AM, et al.
Macromolecules, 46(8), 2963-2968 (2013)
Enol Silyl Ethers via Copper (II)-Catalyzed C-O Bond Formation
Chan DG, et al.
Organic Letters, 13(10), 2778-2781 (2011)
Synthesis of hydrolytically stable porphyrin C-and S-glycoconjugates in high yields
Pasetto P, et al.
Chemical Communications (Cambridge, England), 81-82 (2001)
Direct, Iridium-Catalyzed Enantioselective and Regioselective Allylic Etherification with Aliphatic Alcohols
Ueno S and Hartwig J
Angewandte Chemie (International ed. in English), 47(10), 1928-1931 (2008)
Corinne Nguyen et al.
Journal of medicinal chemistry, 49(14), 4183-4195 (2006-07-11)
We report the discovery of novel uracil-based acyclic compounds as inhibitors of deoxyuridine 5'-triphosphate nucleotidohydrolase (dUTPase), an enzyme involved in nucleotide metabolism that has been identified as a promising target for the development of antimalarial drugs. Compounds were assayed against
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.