295817
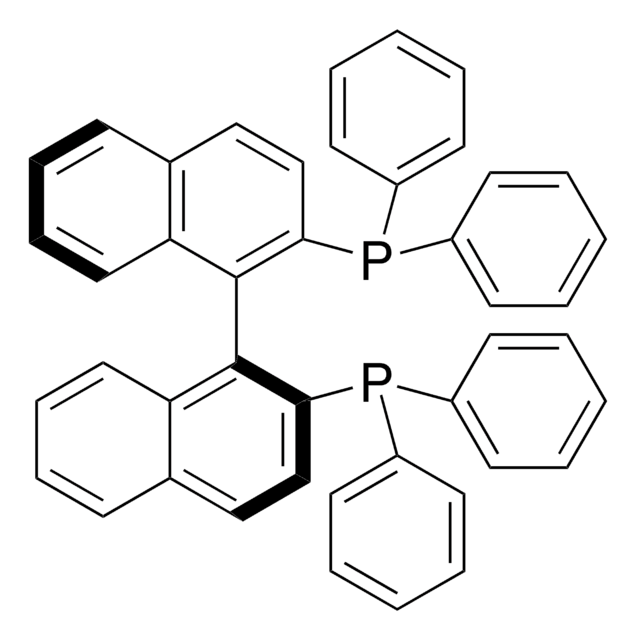
(R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene
97%
동의어(들):
(R)-(+)-(1,1′-Binaphthalene-2,2′-diyl)bis(diphenylphosphine), (R)-(+)-BINAP
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
[(C6H5)2PC10H6-]2
CAS Number:
Molecular Weight:
622.67
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
solid
광학 활성
[α]19/D +233°, c = 0.3 in toluene
광학 순도
ee: 99% (HPLC)
mp
239-241 °C (lit.)
작용기
phosphine
SMILES string
c1ccc(cc1)P(c2ccccc2)c3ccc4ccccc4c3-c5c(ccc6ccccc56)P(c7ccccc7)c8ccccc8
InChI
1S/C44H32P2/c1-5-19-35(20-6-1)45(36-21-7-2-8-22-36)41-31-29-33-17-13-15-27-39(33)43(41)44-40-28-16-14-18-34(40)30-32-42(44)46(37-23-9-3-10-24-37)38-25-11-4-12-26-38/h1-32H
InChI key
MUALRAIOVNYAIW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(R)-(+)-BINAP is a chiral diphosphine ligand that exhibits high enantioselectivity and reactivity in various organic reactions.
(R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene is an axially dissymmetric bis(triaryl)phosphine ligand for asymmetric reactions.
(R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene is an axially dissymmetric bis(triaryl)phosphine ligand for asymmetric reactions.
애플리케이션
Useful ligand for transition metal catalyzed asymmetric reactions, including hydrogenation and disilylation. 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl and its rhodium and ruthenium derivatives are highly selective homogeneous catalysts used for the reduction of aryl ketones, β-keto esters, and α-amino ketones. They have also been used for asymmetric hydrogenation and hydroformylation of olefins, asymmetric Heck reactions, and asymmetric isomerizations of allyls.
Complex with Ag(I) used to catalyze an asymmetric aldol reaction between alkenyl trichloroacetates and aldehydes. Also used with Ag(I) to catalyze an enantioselective hetero-Diels-Alder reaction of azo compounds.
Complex with Ag(I) used to catalyze an asymmetric aldol reaction between alkenyl trichloroacetates and aldehydes. Also used with Ag(I) to catalyze an enantioselective hetero-Diels-Alder reaction of azo compounds.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Journal of Organometallic Chemistry, 692, 550-550 (2007)
Tetrahedron, 50, 335-335 (1994)
Masanori Kawasaki et al.
Journal of the American Chemical Society, 128(51), 16482-16483 (2006-12-21)
This communication describes studies in which an azo hetero-Diels-Alder adduct was furnished in high regio- and enantioselectivity using azopyridine as a reagent and silver as a catalyst. The obtained hetero-Diels-Alder adduct was easily converted to the corresponding chiral 1,4-diamino alcohol.
2,2'-bis (diphenylphosphino)-1, 1'-binaphthyl (binap): A new atropisomeric bis (triaryl) phosphine. synthesis and its use in the Rh (l)-catalyzed asymmetric hydrogenation of a-(acylamino) acrylic acids.
Miyashita A, et al.
Tetrahedron, 40(8), 1245-1253 (1984)
Sci. Synth., 1, 113-264 (2002)
문서
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.






![(S)-4-tert-Butyl-2-[2-(diphenylphosphino)phenyl]-2-oxazoline 97%](/deepweb/assets/sigmaaldrich/product/structures/305/738/18b6aec6-fcf7-4a6d-a8ac-134c41bee9d2/640/18b6aec6-fcf7-4a6d-a8ac-134c41bee9d2.png)