모든 사진(4)
About This Item
실험식(Hill 표기법):
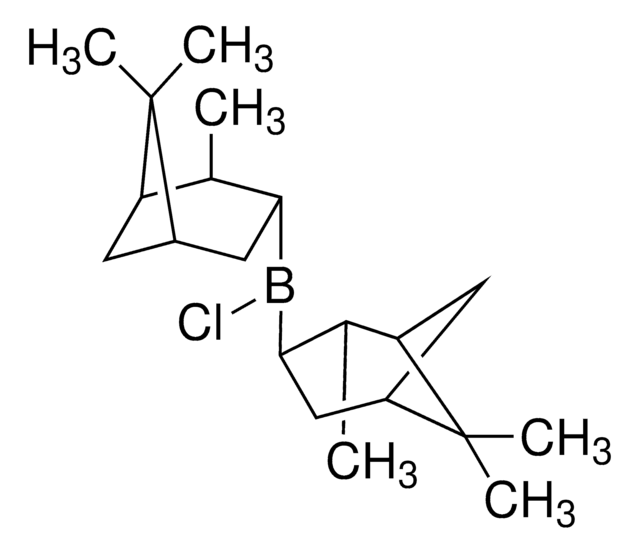
C20H34BCl
CAS Number:
Molecular Weight:
320.75
Beilstein:
5937756
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
형태
solid
Quality Level
mp
52-56 °C (lit.)
SMILES string
C[C@H]1[C@@H](CC2CCC1C2(C)C)B(Cl)[C@@H]3CC4CCC([C@H]3C)C4(C)C
InChI
1S/C20H34BCl/c1-11-15-7-13(19(15,3)4)9-17(11)21(22)18-10-14-8-16(12(18)2)20(14,5)6/h11-18H,7-10H2,1-6H3/t11-,12-,13+,14+,15-,16-,17-,18-/m1/s1
InChI key
PSEHHVRCDVOTID-VMAIWCPRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Both (+)- and (-)-DIP-chloride are used for asymmetric reduction of prochiral ketones and for the preparation of β-amino alcohols.
Reagent used for an enantioselective reduction of (chloroacetyl) benzofurans to chlorohydrins which serve as intermediates for the corresponding epoxides and aminoalcohols. For a short paper on the preparation of DIP halides and their use in stereoselective reductions see Chem. Commun. 1053 (1994).
법적 정보
DIP-Chloride is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Journal of the Chemical Society. Chemical Communications, 1053-1053 (1994)
Tetrahedron Letters, 35, 1511-1511 (1994)
Tetrahedron Asymmetry, 16, 3205-3205 (2005)
Katie M Cergol et al.
Nature protocols, 2(10), 2568-2573 (2007-10-20)
The protocol for the preparation of boron enolates and their subsequent reaction with aldehydes is described, providing convenient access to beta-hydroxy ketones in good yields and with high stereoselectivities. The reaction consists of three steps: first, the ketone is rapidly
Bull. Korean Chem. Soc., 26, 652-652 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.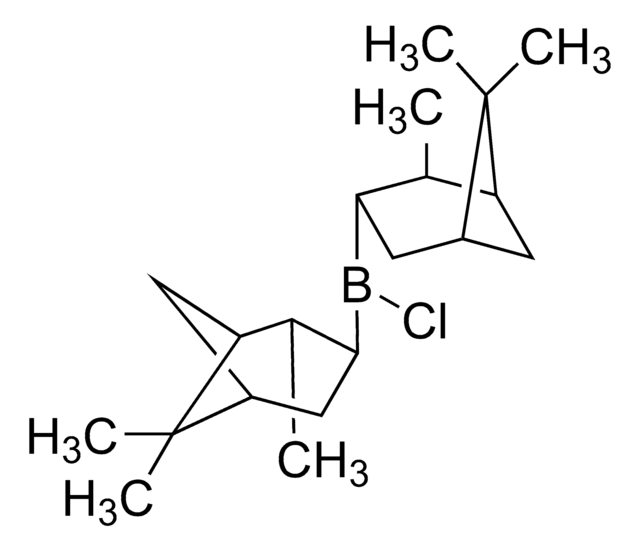




 95%](/deepweb/assets/sigmaaldrich/product/structures/151/609/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1/640/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1.png)
