모든 사진(1)
About This Item
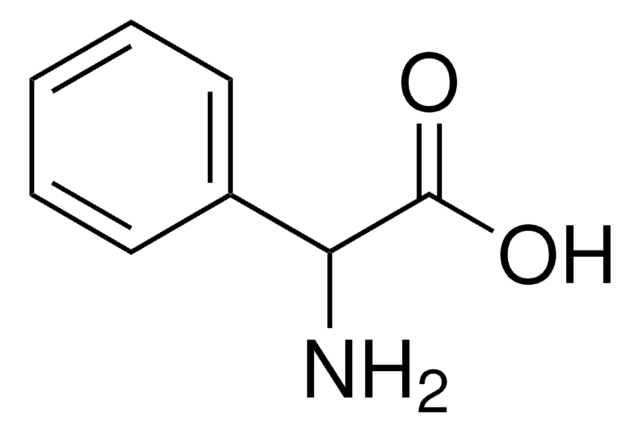
Linear Formula:
C6H5NHCH2COOH
CAS Number:
Molecular Weight:
151.16
Beilstein:
509838
EC Number:
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
반응 적합성
reaction type: solution phase peptide synthesis
mp
121-123 °C (lit.)
응용 분야
peptide synthesis
SMILES string
OC(=O)CNc1ccccc1
InChI
1S/C8H9NO2/c10-8(11)6-9-7-4-2-1-3-5-7/h1-5,9H,6H2,(H,10,11)
InChI key
NPKSPKHJBVJUKB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Y Imai et al.
Dental materials : official publication of the Academy of Dental Materials, 10(4), 275-277 (1994-07-01)
This research was designed to study the effect of water or carboxylic acid monomer on the polymerization of 2-hydroxyethyl methacrylate (HEMA) in order to understand the bonding mechanism of dentin bonding systems using N-phenylglycine (NPG). The polymerization of HEMA in
Zhongqiao Hu et al.
The journal of physical chemistry. B, 113(48), 15851-15857 (2009-11-10)
A microscopic understanding of chiral separation mechanisms in liquid chromatography is significant in the pharmaceutical industry to facilitate the rational design of novel stationary phases and the optimization of separation processes. A molecular simulation study is reported to investigate the
A D Johnston et al.
Journal of dental research, 68(9), 1337-1344 (1989-09-01)
Using bond strength measurements, we investigated a number of related compounds in order to elucidate the role of the surface-active ingredient, N-phenylglycine (NPG), in experimental two-step and three-step bonding protocols resulting in adhesive bonding to dentin. All active compounds identified
R E Webb et al.
Journal of dental research, 70(3), 211-214 (1991-03-01)
Three structurally related substituted amino acids (N-compounds) were studied in a three-step dentin-bonding protocol. The first step of an acidic ferric oxalate solution and the third step of a surface-active comonomer were held constant throughout the study. In the second
G E Schumacher et al.
Journal of dental research, 76(1), 602-609 (1997-01-01)
Effective composite-to-dentin bonding has been achieved by the sequential use of dilute aqueous nitric acid (HNO3) and acetone solutions of N-phenylglycine and a carboxylic acid monomer, e.g., p-PMDM. Both the HNO3 pre-treatment and the surface-initiated polymerization that results from reaction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.