추천 제품
Grade
for chiral derivatization
Quality Level
vapor density
2 (vs air)
vapor pressure
180 mmHg ( 20 °C)
형태
liquid
농도
18 mM in acetone
refractive index
n20/D 1.3602
density
0.79 g/mL at 25 °C
작용기
chloro
저장 온도
2-8°C
InChI
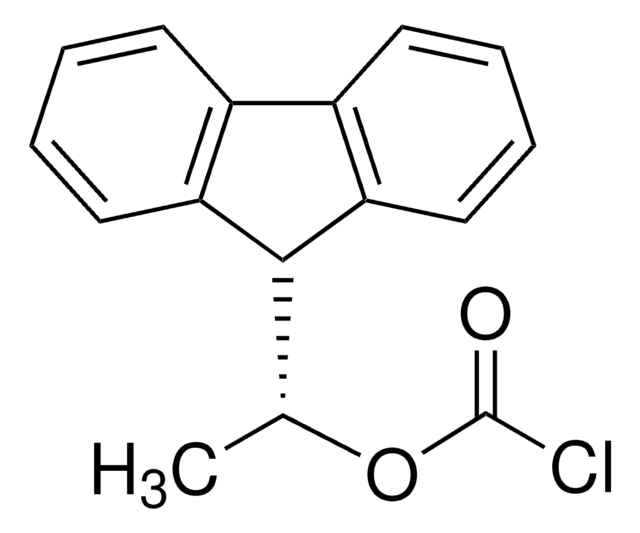
1S/C16H13ClO2/c1-10(19-16(17)18)15-13-8-4-2-6-11(13)12-7-3-5-9-14(12)15/h2-10,15H,1H3
InChI key
SFRVOKMRHPQYGE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(+)-1-(9-Fluorenyl)ethyl chloroformate is a highly fluorescent compound1 commonly used as a chiral derivatizing agent for the separation of racemates prior to reversed-phase HPLC analysis.
애플리케이션
- Chiral analysis of β-methylamino alanine (BMAA) enantiomers: Details the use of (+)-1-(9-fluorenyl)-ethyl chloroformate (FLEC) for derivatization followed by LC-MS/MS analysis, improving the understanding of amino acids′ stereochemistry (Zurita et al., 2019).
- Enantioselective micellar electrokinetic chromatography of dl‐amino acids: Utilizes (+)-1-(9-fluorenyl)ethyl chloroformate derivatization combined with UV-induced fluorescence detection to analyze amino acids, enhancing analytical methodologies (Prior et al., 2018).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
1.4 °F
Flash Point (°C)
-17 °C
이미 열람한 고객
Reversed-phase high-performance liquid chromatographic analysis of atenolol enantiomers in plasma after chiral derivatization with (+)-1-(9-fluorenyl) ethyl chloroformate.
Rosseel MT, et al.
Journal of Chromatography. B, Biomedical Applications, 568(1), 239-245 (1991)
Zeineb Aturki et al.
Electrophoresis, 25(4-5), 607-614 (2004-02-26)
The indirect resolution of five beta-adrenoreceptor blocking agents (propranolol, oxprenolol, pindolol, metoprolol, and atenolol) using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate (FLEC), and capillary electrochromatography (CEC) is reported. Three octadecylsilanized (ODS) silica gel-based stationary phases, differing in particle diameter and carbon
A Roux et al.
Journal of chromatography, 570(2), 453-461 (1991-10-04)
A method for the determination of the R-(+) and S-(-) enantiomers of propranolol in blood was developed. After extraction with heptane-isopentanol and derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate, excess reagent was removed using solid-phase extraction. The enantiomers were separated on an achiral
Radu-Cristian Moldovan et al.
Journal of chromatography. A, 1513, 1-17 (2017-08-02)
Over the last 30years, (±)-1-(9-fluorenyl)ethyl chloroformate ((±)-FLEC) was used as a chiral derivatizing agent in various analytical applications involving a wide range of endogenous, pharmaceutical and environmentally relevant molecules. This comprehensive review aims to present all the significant aspects related
Sascha Freimüller et al.
Journal of pharmaceutical and biomedical analysis, 30(2), 209-218 (2002-08-23)
An indirect enantioseparation method for robust and precise determination of D-Carnitine (D-C) in L-Carnitine (L-C) in the range of 0.1-1.0% is presented. The method is based on derivatization of Carnitine with (+)-[1-(9-fluorenyl)-ethyl]-chloroformate ((+)-FLEC). The two diastereomers are subsequently separated of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.