모든 사진(1)
About This Item
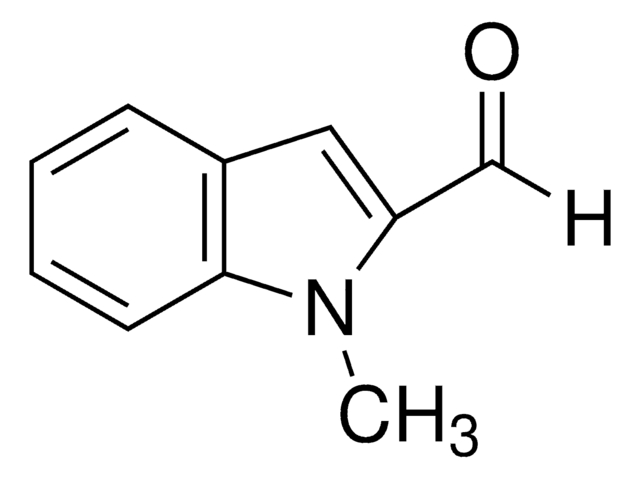
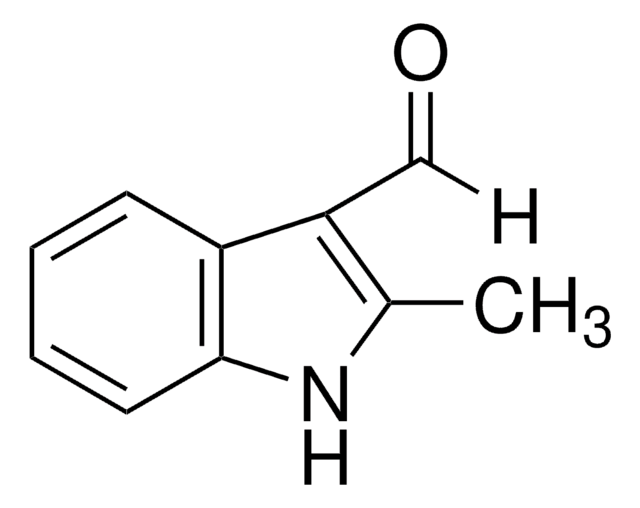
실험식(Hill 표기법):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
70-72 °C (lit.)
작용기
aldehyde
SMILES string
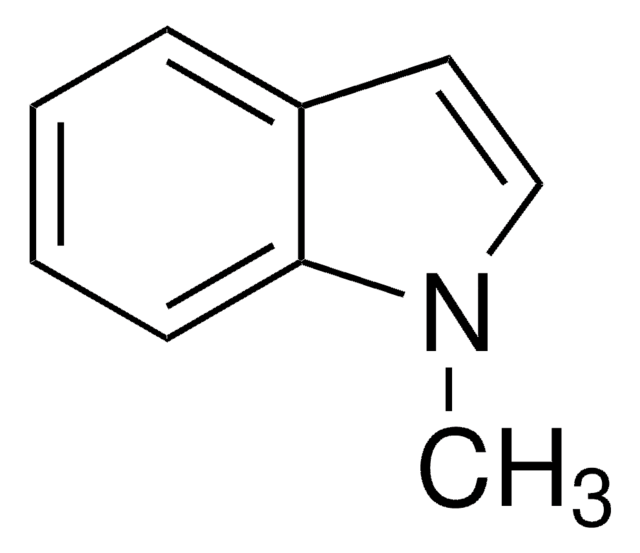
Cn1cc(C=O)c2ccccc12
InChI
1S/C10H9NO/c1-11-6-8(7-12)9-4-2-3-5-10(9)11/h2-7H,1H3
InChI key
KXYBYRKRRGSZCX-UHFFFAOYSA-N
일반 설명
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
애플리케이션
1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
- Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions
- Reactant for synthesis of quinolinones via three-component Ugi reaction
- Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture
- Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins
- Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent
- Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Preliminary analysis of the 1H-and 13C-NMR spectra of poly (3-vinyl-1-methylindole).
Trumbo DL.
Polymer Bull., 37(1), 75-80 (1996)
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 60(Pt 3), o217-o218 (2004-03-09)
The title compound, C16H12N2S, has been synthesized by base-catalyzed condensation of 1-methylindole-3-carboxaldehyde with thiophene-3-acetonitrile. The product assumes an approximately planar Z configuration. The molecule has a thienyl-ring flip disorder.
Wagee A Yehye et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 9), o1824-o1824 (2008-01-01)
In the crystal structure of the title Schiff-base, C(20)H(21)N(3)O(4), the amino group forms an N-H⋯O hydrogen bond to the acetyl group of an adjacent mol-ecule, forming a zigzag chain. The 2-hydr-oxy group is inter-nally hydrogen bonded to the amido group
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.