추천 제품
Quality Level
분석
97%
mp
200-201 °C (lit.)
작용기
aldehyde
SMILES string
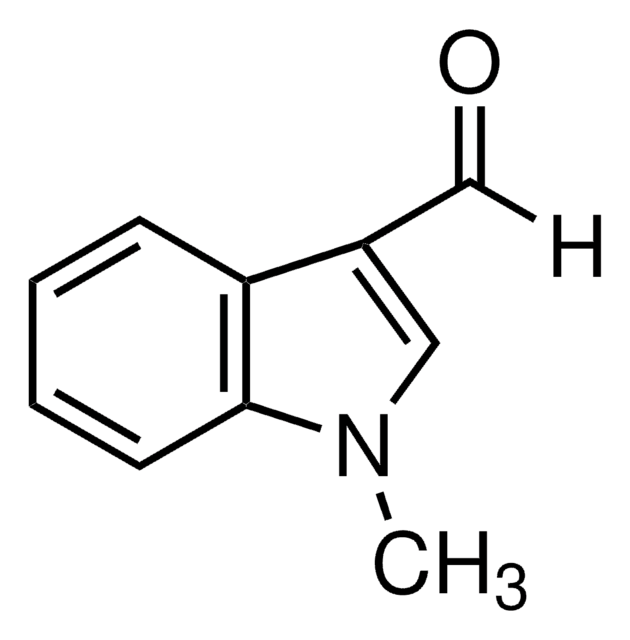
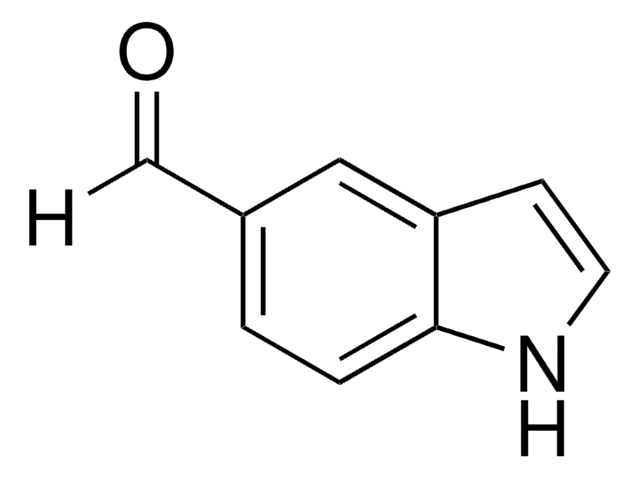
Cc1[nH]c2ccccc2c1C=O
InChI
1S/C10H9NO/c1-7-9(6-12)8-4-2-3-5-10(8)11-7/h2-6,11H,1H3
InChI key
CYZIVXOEJNAIBS-UHFFFAOYSA-N
일반 설명
Oxidative activation of 2-methylindole-3-carboxaldehyde via N-heterocyclic carbene organocatalysis generates heterocyclic ortho-quinodimethane as a key intermediate.
애플리케이션
2-Methylindole-3-carboxaldehyde has been used in the preparation of 1-phenylsulfonyl-2-methylindole-3-carboxaldehyde.
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Fluorescent sensors (BODIPY)
- Antimicrobial agents against methicillin-resistant Staphylococcus aureus
- G protein-coupled receptor CRTh2 antagonists
- Inhibitors of PI3 kinase-α
- Antitubercular agents
- Anti-inflammatory agents
- Mycobacterium tuberculosis protein tyrosine phosphatase B
- Glucocorticoid receptor ligands
- Agents stimulating neurite outgrowth
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Xingkuan Chen et al.
Angewandte Chemie (International ed. in English), 52(42), 11134-11137 (2013-09-17)
Aryl aldehyde activation: Oxidative activation of 2-methylindole-3-carboxaldehyde (I) through N-heterocyclic carbene (NHC) organocatalysis generates heterocyclic ortho-quinodimethane (II) as a key intermediate. This intermediate then undergoes formal [4+2] cycloaddition with trifluoromethyl ketones or isatins to form polycyclic lactones containing a quaternary
G Chakkaravarthi et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o542-o542 (2008-01-01)
In the title compound, C(16)H(15)NO(3)S, the plane of the phenyl ring forms a dihedral angle of 80.37 (8)° with the indole ring system. The crystal packing is stabilized by weak O-H⋯O hydrogen bonds which link the mol-ecules into infinite chains along
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.