추천 제품
Quality Level
분석
≥99%
bp
253-254 °C (lit.)
mp
51-54 °C (lit.)
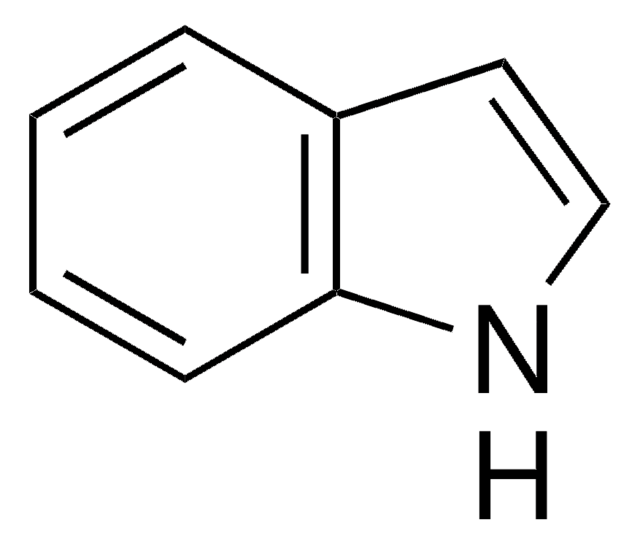
SMILES string
c1ccc2[nH]ccc2c1
InChI
1S/C8H7N/c1-2-4-8-7(3-1)5-6-9-8/h1-6,9H
InChI key
SIKJAQJRHWYJAI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Indole is a nitrogen-containing heterocycle used in the total synthesis of compounds such as goniomitine, (−)-isatisine A, and (±)-aspidospermidine.
- It is used as an electron donor moiety in synthesizing dyes for organic photovoltaics.
- It can also be used in the preparation of indole based conjugated small molecules for nonlinear optics applications.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
249.8 °F - closed cup
Flash Point (°C)
121 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Catalytic selective cyclizations of aminocyclopropanes: formal synthesis of aspidospermidine and total synthesis of goniomitine
De Simone F, et al.
Angewandte Chemie (International ed. in English), 122(33), 5903-5906 (2010)
Regioselective Inter-and Intramolecular Formal [4+ 2] Cycloaddition of Cyclobutanones with Indoles and Total Synthesis of (?)-Aspidospermidine
Kawano M, et al.
Angewandte Chemie (International ed. in English), 125(3), 940-944 (2013)
Impact of Thermal Annealing on Organic Photovoltaic Cells Using Regioisomeric Donor-Acceptor-Acceptor Molecules
Zhang T, et al.
ACS Applied Materials & Interfaces, 9(30), 25418-25425 (2017)
Synthesis and nonlinear optical properties of novel conjugated small molecules based on indole donor
Liu J, et al.
Journal of Molecular Structure, 1165(30), 223-227 (2018)
Total Synthesis of (−)-Isatisine A
Zhang X, et al.
Angewandte Chemie (International ed. in English), 50(27), 6164-6166 (2011)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.