추천 제품
일반 설명
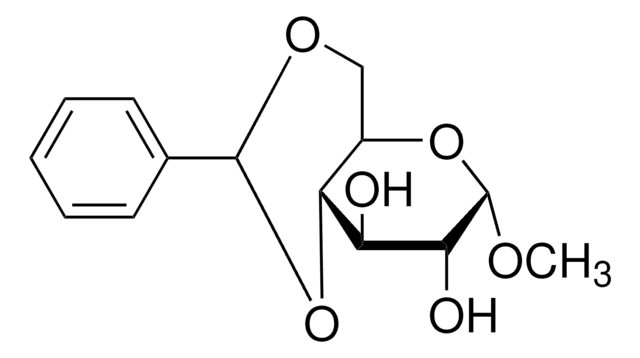
Benzaldehyde dimethyl acetal is an organic building block. Pyridinium tosylate-catalyzed acetal exchange reaction between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-α-D-glucofuranose is reported to afford 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranose. The kinetics of the hydrolysis of benzaldehyde dimethyl acetal over amberlite IR-120 has been studied using a circulated batch reactor in dioxane. One-pot tandem conversion of benzaldehydedimethylacetal to trans-1-nitro-2-phenylethylene has been reported.
애플리케이션
Benzaldehyde dimethyl acetal is suitable for use in the synthesis of 4,6-dihydroxy sugar, required for the total synthesis of Porphyromonas gingivalis 381 derived lipid A. It may be used in the preparation of 1-O-methyl-2,3-di-O-galloyl-β-D-glucose.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
156.2 °F - closed cup
Flash Point (°C)
69 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Mesoporous silica with site-isolated amine and phosphotungstic acid groups: a solid catalyst with tunable antagonistic functions for one-pot tandem reactions.
N Raveendran Shiju et al.
Angewandte Chemie (International ed. in English), 50(41), 9615-9619 (2011-09-16)
T Ogawa et al.
FEMS immunology and medical microbiology, 28(4), 273-281 (2000-07-13)
A synthetic lipid A of Porphyromonas gingivalis strain 381 (compound PG-381), which is similar to its natural lipid A, demonstrated no or very low endotoxic activities as compared to Escherichia coli-type synthetic lipid A (compound 506). On the other hand
K Akerfeldt et al.
Carbohydrate research, 158, 137-145 (1986-12-15)
Pyridinium tosylate-catalyzed acetal exchange between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-alpha-D-glucofu ranose was investigated as an alternative to the original procedure of Brigl and Grüner (condensation of a D-glucose triol with benzaldehyde under zinc halide catalysis) for synthesis of 3,5-O-benzylidene-1,2-O-isopropylidene-alpha-D-glucofuranose. The
M Toda et al.
Bioscience, biotechnology, and biochemistry, 65(3), 542-547 (2001-05-02)
The clove ellagitannins and their related polygalloyl-glucoses inhibited maltase activity of rat intestinal alpha-glucosidases. The structure-activity relationship study of those galloylglucoses, varying the extent of galloylation on the glucose core, with the ellagitannins, indicated that an increasing number of galloyl
Kinetics of hydrolysis of benzaldehyde dimethyl acetal over Amberlite IR-120.
Altiokka MR and Hosgun HL.
Industrial & Engineering Chemistry Research, 46(4), 1058-1062 (2007)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.