모든 사진(1)
About This Item
Linear Formula:
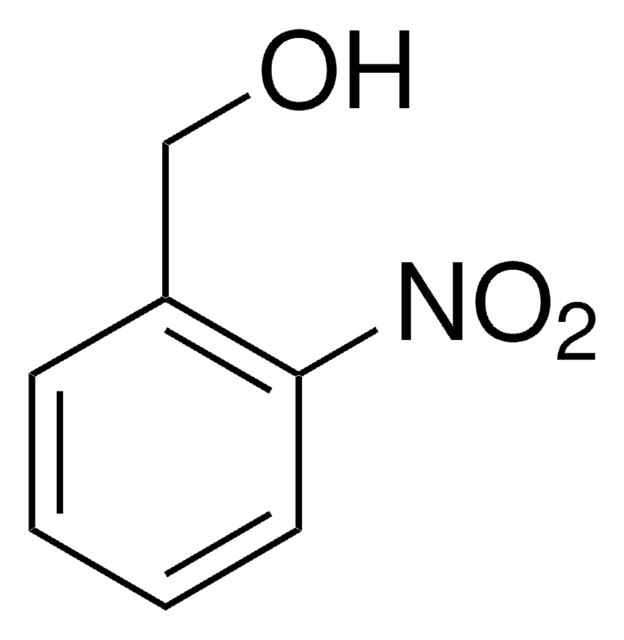
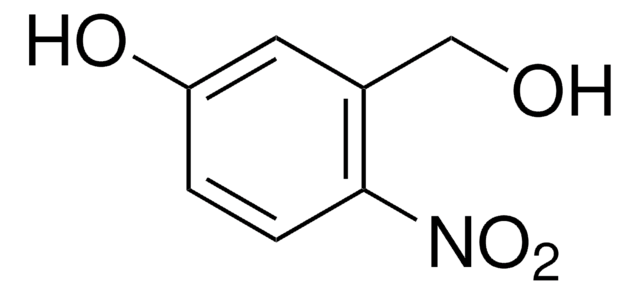
O2NC6H2(OCH3)2CH2OH
CAS Number:
Molecular Weight:
213.19
Beilstein:
1880093
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
mp
145-148 °C (lit.)
SMILES string
COc1cc(CO)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H11NO5/c1-14-8-3-6(5-11)7(10(12)13)4-9(8)15-2/h3-4,11H,5H2,1-2H3
InChI key
WBSCOJBVYHQOFB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-Nitroveratryl Alcohol) is 2-nitrobenzyl alcohol derivative. It has been reported to be one of the oxidation products of veratryl (3,4-dimethoxybenzyl) alcohol by lignin peroxidase (isolated from Phanerochaete chrysosporium).
애플리케이션
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-nitroveratryl alcohol) is suitable reagent used in the synthesis of 4,5-dimethoxy-2-nitrobenzyl methacrylate, a photolabile monomer and 2-(4-((4-(4,5-dimethoxy-2-nitrobenzyloxy)phenyl)cyclohexylidene)methyl)phenoxy)-N,N-dimethylethanamine, a caged cyclofen-OH ligand.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
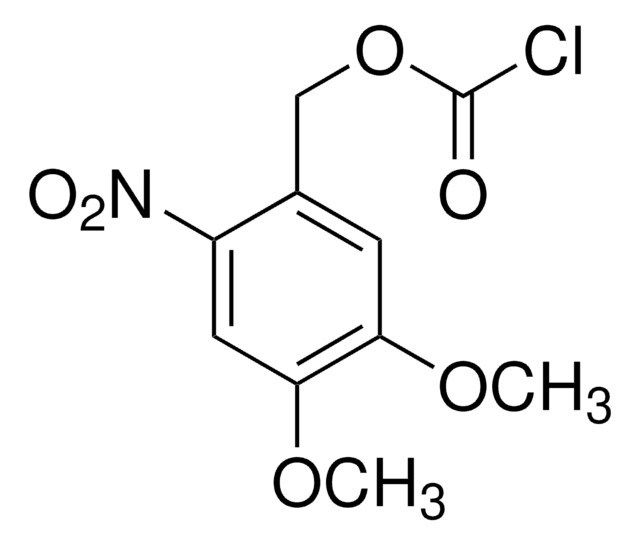
- 1-[[(chlorocarbonyl)oxy]methyl]-4,5-dimethoxy-2-nitrobenzene
- bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol, a photolabile protecting group
- optically-sensitive monomer
- nitroveratryl (NV) protected α-hydroxyacetic acid (αG) (NV-αG-OH), required in the preparation of nitroveratryl (NV) protected cyanomethyl (CM) ester of α-hydroxyacetic acid (αG) (NV-αG-CM)
- 4,5-dimethoxy-2-nitrobenzyl p-nitro-phenylcarbonate
- 6-nitroveratryloxycarbonyl chloride (NVOCCl), a reagent used in the protection of amino function in amino sugars
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Andrew A Brown et al.
Langmuir : the ACS journal of surfaces and colloids, 25(3), 1744-1749 (2009-01-10)
The use of photolabile protecting groups (PGs) as a means to create latent hydrophilic surfaces is presented. Naturally hydrophobic PGs, based on o-nitrobenzyl chemistry, are used on polymer side chains, poised for cleavage upon exposure to UV light. Removal of
Nadezda Fomina et al.
Journal of the American Chemical Society, 132(28), 9540-9542 (2010-06-24)
A new light-sensitive polymer containing multiple light-sensitive triggering groups along the backbone and incorporating a quinone-methide self-immolative moiety was developed and formulated into nanoparticles encapsulating a model pharmaceutical Nile Red. Triggered burst release of the payload upon irradiation and subsequent
Bis (4, 5-dimethoxy-2-nitrophenyl) ethylene glycol: a new and efficient photolabile protecting group for aldehydes and ketones.
Kantevari S, et al.
Tetrahedron, 61(24), 5849-5854 (2005)
Photosensitive protecting groups of amino sugars and their use in glycoside synthesis. 2-nitrobenzyloxycarbonylamino and 6-nitroveratryloxycarbonylamino derivatives.
Amit B, et al.
The Journal of Organic Chemistry, 39(2), 192-196 (1974)
Peptide backbone mutagenesis of putative gating hinges in a potassium ion channel.
Yasuo Nagaoka et al.
Chembiochem : a European journal of chemical biology, 9(11), 1725-1728 (2008-06-11)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.