추천 제품
Quality Level
분석
≥99.0% (T)
양식
solid
무기 잔류물
≤0.1%
mp
121-123 °C (lit.)
123-126 °C
solubility
hot water: soluble 0.5 g/10 mL, clear, colorless to faintly yellow
SMILES string
CN1C(=O)CC(=O)N(C)C1=O
InChI
1S/C6H8N2O3/c1-7-4(9)3-5(10)8(2)6(7)11/h3H2,1-2H3
InChI key
VVSASNKOFCZVES-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1,3-Dimethylbarbituric acid (1,3-Dimethyl-2,4,6(1H,3H,5H)-pyrimidinetrione) is an active methylene compound. It undergoes hollow Pd6 water-soluble cage, [{(tmen)Pd}6(timb)4](NO3)12 (tmen= N,N,N′,N′-tetramethylethylenediamine, timb=1,3,5-tris(1-imidazolyl)benzene)-catalyzed Knoevenagel condensation reaction with pyrene-1-carboxaldehyde. It undergoes self-sorted Pd7 molecular boat having an internal nanocavity (catalyst)-assisted Knoevenagel condensation reaction with various aromatic aldehydes. It has been synthesized by reacting 1,3-dimethylurea, malonic acid and acetic anhydride in acetic acid. It is widely used for the synthesis of various synthetic intermediates and heterocyclic compounds.
애플리케이션
1,3-Dimethylbarbituric acid may be used in the following studies:
- Enantioselective synthesis of isochromene pyrimidinedione derivatives having five stereocenters, via one-pot Michael-Knoevenagel condensation-inverse-electron-demand hetero-Diels-Alder reaction.
- Synthesis of 5-aryl-6-(alkyl- or aryl-amino)-1,3-dimethylfuro [2,3-d]pyrimidine derivatives.
- Microwave promoted indirect functionalization of alcohols, via spirocyclisation employing a sequential one-pot Ir(III)/Pd(0) catalyzed process.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Shahram Lotfi et al.
Materials science & engineering. C, Materials for biological applications, 76, 153-160 (2017-05-10)
Electrochemical oxidation of trimipramine in the absence and presence of 1,3 dimethyl barbituric acid as a nucleophile in aqueous solution has been studied using cyclic voltammetry and controlled-potential coulometry electrolysis. Voltammetric studies of electro-oxidation of trimipramine were realized in a
1, 3-Dimethylbarbituric Acid
Argintaru OA.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2011)
Christian Löfberg et al.
Chemical communications (Cambridge, England), (48)(48), 5000-5002 (2006-12-06)
Microwave assisted indirect functionalization of alcohols with 1,3-dimethylbarbituric acid followed by spirocyclisation employing a sequential one-pot Ir(III)/Pd(0) catalysed process, involving the formation of three new C-C bonds, one spirocyclic ring and one di- or tri-substituted exocyclic alkene, is described.
Bor-Cherng Hong et al.
Organic letters, 14(2), 448-451 (2011-12-27)
Synthesis of isochromene pyrimidinedione derivatives having five stereocenters has been achieved by a one-pot Michael-Knoevenagel condensation-inverse-electron-demand hetero-Diels-Alder reaction of α, β-unsaturated aldehydes, olefinic nitroalkanes, and 1,3-dimethylbarbituric acid via a one-pot strategy with excellent diastereo- and enantioselectivities (up to 99% ee).
Burkhard Knopf et al.
Environmental science and pollution research international, 28(13), 16244-16252 (2020-12-18)
Cyanide compounds are naturally emitted into the environment in low levels by degradation processes or emitted from anthropogenic sources. In surface water, complex cyanide compounds as well as "free cyanide" are present. The latter term covers hydrogen cyanide and cyanide
문서
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.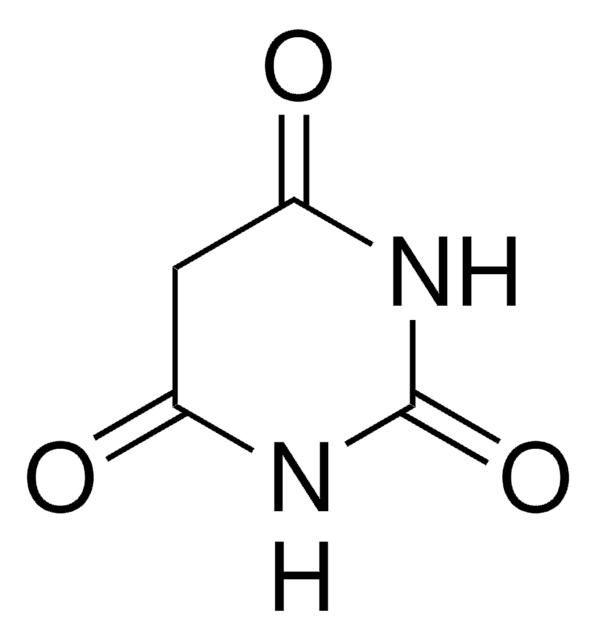

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)