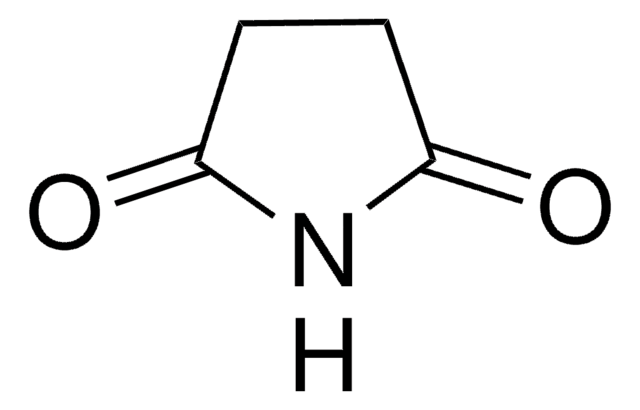
추천 제품
Quality Level
분석
≥99.5% (HPLC)
≥99.5%
형태
solid
품질
for spectrophotometric det. of cyanide
기술
UV/Vis spectroscopy: suitable
무기 잔류물
≤0.05%
mp
248-252 °C (dec.) (lit.)
음이온 미량물
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤500 mg/kg
양이온 미량물
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
Mg: ≤10 mg/kg
Mn: ≤5 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
SMILES string
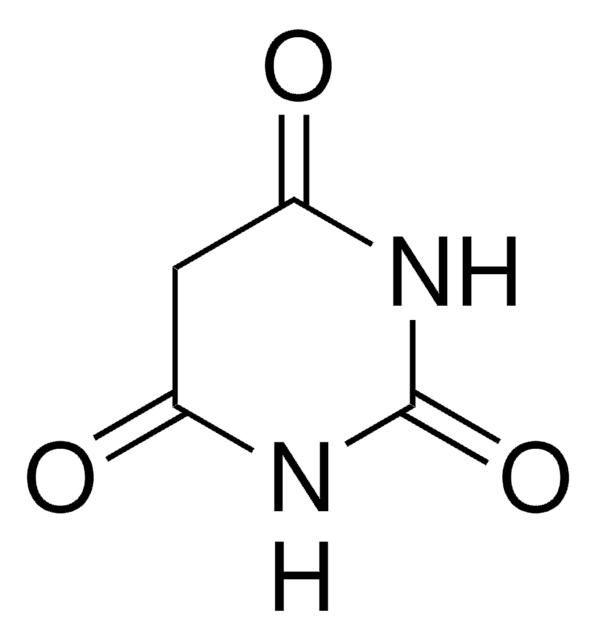
O=C1CC(=O)NC(=O)N1
InChI
1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9)
InChI key
HNYOPLTXPVRDBG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Barbituric acid is also known as malonurea or 6-hydroxyuracil, it is an odourless powder and is soluble in water. It has a very high pharmacological activity and can be used to synthesize addition derivatives of it, to be used in novel drug discovery. It basically acts on the central nervous system (CNS) depressants, thereby possessing a wide range from mild sedation to total anaesthesia.
애플리케이션
Barbituric acid with aromatic aldehydes was used in an experimental study, meant to demonstrate the increased efficiency of Knoevenagel condensation reaction for barbituric acid and various aromatic aldehydes on basic alumina, in the absence of organic solvents under microwave irradiation. It may also be used in electrochemical oxidation of iodine, using cyclic voltammetry and controlled-potential coulometry.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
302.0 °F - closed cup
Flash Point (°C)
150.00 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Electrochemical study of iodide in the presence of barbituric acid. Application to coulometric titration of barbituric acid.
Nematollahi D and Hesari M
Microchemical Journal, Devoted to the Application of Microtechniques in All Branches of Science, 70 (1), 7-11 (2001)
Bioactive Heterocyclic Compound Classes.
Clemens L and Dinges J.
Pharmaceutics, 24-27 (2012)
Microwave enhanced knoevenagel condensation of barbituric acid with aromatic aldehydes on basic alumina.
Khalafi NA and Hashemi A.
Iranian Journal of Chemistry and Chemical Engineering, 20 (1), 9-11 (2001)
Thomas Gelbrich et al.
Acta crystallographica. Section C, Crystal structure communications, 66(Pt 1), o55-o58 (2010-01-06)
Both title structures exhibit essentially planar barbiturate rings. The crystal structure of enallylpropymal (5-allyl-5-isopropyl-1-methylbarbituric acid), C(11)H(16)N(2)O(3), is composed of centrosymmetric N-H...O hydrogen-bonded dimers, while 1,5-di(but-2-enyl)-5-ethylbarbituric acid, C(14)H(20)N(2)O(3), forms N-H...O hydrogen-bonded helical chains. Each of the ten known crystal structures of
Liang Ma et al.
European journal of medicinal chemistry, 46(6), 2003-2010 (2011-03-25)
Forty-four barbituric acid or thiobarbituric acid derivatives were synthesized and evaluated for their effects on adipogenesis of 3T3-L1 adipocytes by measuring the expression of adiponectin in vitro. Four compounds (3a, 3o, 3s, 4t) were found to increase the expression of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.