40766
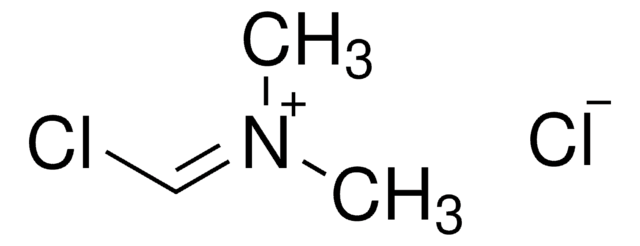
N,N-Dimethylmethyleneiminium chloride
≥95.0% (AT)
동의어(들):
Böhme′s salt, Böhme′s salt, Dimethylformiminium chloride, Methylenedimethylammonium chloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH2=N+(CH3)2Cl-
CAS Number:
Molecular Weight:
93.56
Beilstein:
505955
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95.0% (AT)
양식
powder
반응 적합성
reaction type: C-C Bond Formation
mp
146-148 °C (lit.)
작용기
amine
SMILES string
[Cl-].C[N+](C)=C
InChI
1S/C3H8N.ClH/c1-4(2)3;/h1H2,2-3H3;1H/q+1;/p-1
InChI key
ZJTROANVDZIEGB-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
N,N-Dimethylmethyleneiminium chloride (Böhme′s salt) is a dimethylaminomethylating agent that can be used as one of the key reagents in the following applications:
- Electrophilic aminomethylation of aldehydes and ketones to synthesize corresponding Mannich products, also known as Mannich reaction.
- Preparation of dihydronaphthyridinones as HIV-1 integrase inhibitors.
- Preparation of cyanotetrahydrooxo-β-carbolines via cyclocondensation of cyanomethyl indole-2-carboxylate with ammonia.
- Asymmetric synthesis of phalarine via stereospecific Pictet-Spengler cyclocondensation and traceless chirality transfer from L-tryptophan.
- Synthesis of functionalized diarylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX).
- Synthesis of (±)-phalarine with the rearrangement of an azaspiroindolenine and Gassman oxindole preparation as key steps.
Reacant for:
Preparation of dihydronaphthyridinones as HIV-1 integrase inhibitors
Mannich reactions
Preparation of cyanotetrahydrooxo-ß-carbolines via cyclocondensation reactions
Asymmetric synthesis of phalarine via stereospecific Pictet-Spengler cyclocondensation and traceless chirality transfer from L-tryptophan
Synthesis of functionalized diarylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX)
Synthesis of (±)-phalarine with the rearrangement of an azaspiroindolenine and Gassman oxindole preparation as key steps
Preparation of dihydronaphthyridinones as HIV-1 integrase inhibitors
Mannich reactions
Preparation of cyanotetrahydrooxo-ß-carbolines via cyclocondensation reactions
Asymmetric synthesis of phalarine via stereospecific Pictet-Spengler cyclocondensation and traceless chirality transfer from L-tryptophan
Synthesis of functionalized diarylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX)
Synthesis of (±)-phalarine with the rearrangement of an azaspiroindolenine and Gassman oxindole preparation as key steps
기타 정보
"Mannich-reagent" for direct use; higher yields of purer products were achieved in shorter times compared with the classical "Mannich reaction"; in-situ preparation of the reactive triflate with TMS-triflate
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
179.6 °F - closed cup
Flash Point (°C)
82 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Arylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) or as dual inhibitors of mPGES-1 and 5-lipoxygenase (5-LOX).
Liedtke A J, et al.
Journal of Medicinal Chemistry, 52(15), 4968-4972 (2009)
otal Synthesis of Phalarine.
Li C, et al.
Angewandte Chemie (International Edition in English), 46(9), 1448-1450 (2007)
Design and synthesis of novel N-hydroxy-dihydronaphthyridinones as potent and orally bioavailable HIV-1 integrase inhibitors.
Johnson T W, et al.
Journal of Medicinal Chemistry, 54(9), 3393-3417 (2011)
A versatile synthesis of 3-substituted 4-cyano-1, 2, 3, 4-tetrahydro-1-oxo-β-carbolines.
Huber K, et al.
Synthesis, 2010(22), 3849-3854 (2010)
H.-U. Reissig et al.
Liebigs Ann. Chem., 1914-1914 (1986)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.