456055
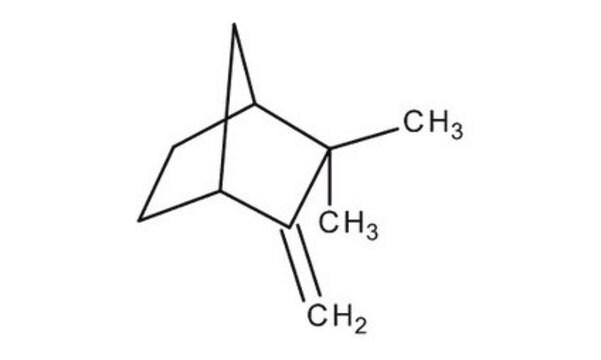
Camphene
95%
동의어(들):
(±)-Camphene, 2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane, 2,2-Dimethyl-3-methylenenorbornane, 2,2-Dimethyl-3-methylidenebicyclo[2.2.1]heptane, 2-Methylene-3,3-dimethylbicyclo[2.2.1]heptane, 3,3-Dimethyl-2-methylenenorbornane, 3,3-Dimethyl-2-methylenenorcamphane, DL-Camphene
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C10H16
CAS Number:
Molecular Weight:
136.23
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Camphene is abicyclic monoterpene and a constituent of many essential oils derived fromvarious plants such as Alpinia speciosa, Xanthium strumariumleaves, needles of Pinus uncinata, and Pinus uliginosa.(5)
애플리케이션
Camphene can be used as a reactant to synthesize:
- Isobornyl carboxylates by silica-supported tungstophosphoric acid-catalyzed liquid-phase esterification of C2-C6 fatty acids.
- Hydroaminated camphene via intermolecular anti-Markovnikov hydroamination reaction with N-hydroxyphthalimide and triethyl phosphite in the presence of dilauroyl peroxide as an initiator.
- Camphene oxide via methyltrioxorhenium-catalyzed epoxidation in the presence of H2O2 as an oxidant and pyrazole as a Lewis base adduct.
- Isobornyl alkyl ethers using alcohols via cation exchange resin-catalyzed alkoxylation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 2
Flash Point (°F)
78.8 °F - DIN 51755 Part 1
Flash Point (°C)
26 °C - DIN 51755 Part 1
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Dendritic growth kinetics and structure II. Camphene.
Rubinstein ER and Glicksman ME.
Journal of Crystal Growth, 112(1), 97-110 (1991)
Radosław Bonikowski et al.
Natural product communications, 10(2), 371-373 (2015-04-30)
The compositions of mountain pine (Pinus uncinata) and peat-bog pine (P. uliginosa) needle essential oils were investigated. Enantiomeric compositions of selected monoterpene hydrocarbons were also examined. Respectively, fifty-three and seventy-six components of the essential oils were identified using GC-MS and
Javad Sharifi-Rad et al.
Molecules (Basel, Switzerland), 20(4), 7034-7047 (2015-04-22)
The chemical composition of the essential oil (EO) from fresh cocklebur (Xanthium strumarium L.) leaves was investigated by GC-MS. The antimicrobial activity of the EO was tested against Gram-positive and Gram-negative bacteria and fungi. Scolicidal activity was assayed against Echinococcus
Lakshmi Vasireddy et al.
PloS one, 13(8), e0201835-e0201835 (2018-08-03)
Members of the Burkholderia cepacia complex (Bcc) are an important cause of opportunistic or nosocomial infections that may be hard to treat due to a high incidence of multidrug resistance. We characterised a collection of 51 clinical isolates from this
Interactions with β-cyclodextrin as a way for encapsulation and separation of camphene and fenchene.
Magdalena Ceborska et al.
Carbohydrate polymers, 91(1), 110-114 (2012-10-10)
The separation of isomeric monoterpenes, camphene and fenchene by complexation with β-cyclodextrin is presented. Both of the monoterpenes form complexes with β-cyclodextrin (as shown by both gas chromatography and (1)H NMR) with similar stability constants nevertheless it is possible to
프로토콜
GC Analysis of Sweet Orange Essential Oil on SLB®-5ms (10 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.