481858
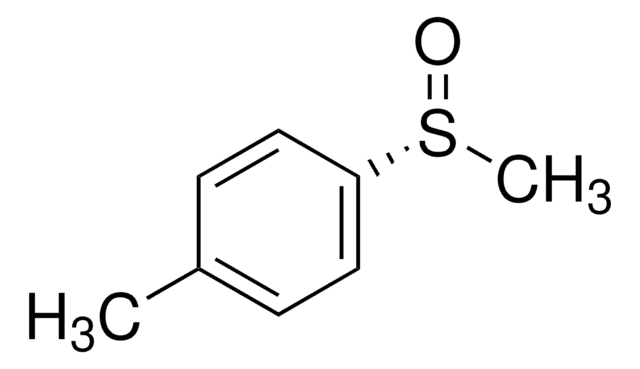
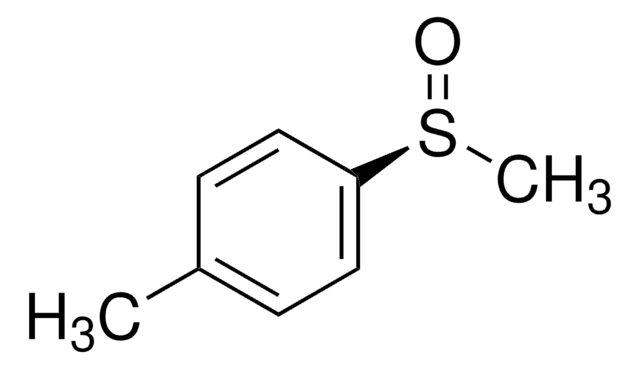
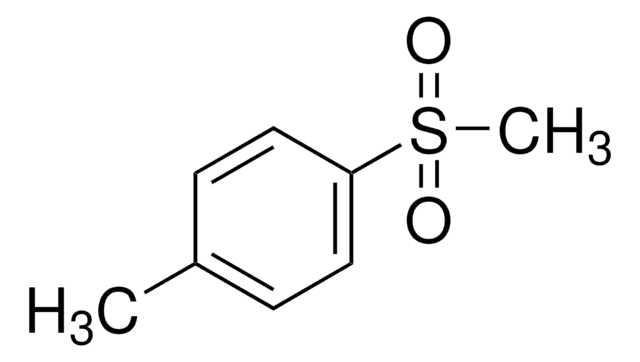
Methyl p-tolyl sulfoxide
97%
동의어(들):
(±)-Methyl p-toluene sulfoxide, (±)-p-Tolyl methyl sulfoxide, 1-Methanesulfinyl-4-methylbenzene, 1-Methyl-4-(methylsulfinyl)benzene, 4-(Methylsulfinyl)toluene, 4-Methylphenyl methyl sulfoxide, 4-Toluene methyl sulfoxide, Methyl 4-methylphenyl sulfoxide, p-Methylphenyl methyl sulfoxide, p-Tolyl methyl sulfoxide
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
CH3C6H4S(O)CH3
CAS Number:
Molecular Weight:
154.23
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
bp
113-114 °C/2 mmHg (lit.)
mp
44-46 °C (lit.)
작용기
sulfoxide
SMILES string
Cc1ccc(cc1)S(C)=O
InChI
1S/C8H10OS/c1-7-3-5-8(6-4-7)10(2)9/h3-6H,1-2H3
InChI key
FEVALTJSQBFLEU-UHFFFAOYSA-N
일반 설명
Methyl p-tolyl sulfoxide (MTSO), an alkyl-substituted p-tolyl sulfoxide, is a Lewis base. It behaves as an activator of silicon tetrachloride that is employed in aza-Diels-Alder reaction.
애플리케이션
Methyl p-tolyl sulfoxide may be used as a catalyst in the preparation of homoallylic alcohols by the allylation of aldehydes with allyltrichlorosilane. It may be used as a ligand in the synthesis of molybdenum chlorocomplexes.
주의사항
Low melting solid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Addition compounds of MoO2Cl2 with chiral sulfoxides. First molecular structures of dioxomolybdenum complexes bearing chiral non-racemic sulfoxide as ligand.
Pedrosa MR, et al.
Inorgorganica Chimica Acta, 363(13), 3158-3164 (2010)
Luisa A Denkel et al.
PloS one, 6(11), e26974-e26974 (2011-11-11)
Production of reactive oxygen species represents a fundamental innate defense against microbes in a diversity of host organisms. Oxidative stress, amongst others, converts peptidyl and free methionine to a mixture of methionine-S- (Met-S-SO) and methionine-R-sulfoxides (Met-R-SO). To cope with such
A New Approach to Di-and Tetrasubstituted 2,3-Dihydropyridin-4(1H)-ones through Aza-Diels-Alder Reaction Promoted by Silicon Tetrachloride.
Peduto A, et al.
Synthesis, 4, 0643-0649 (2009)
Asymmetric allylation of aldehydes with allyltrichlorosilane promoted by chiral sulfoxides.
Massa A, et al.
Tetrahedron Letters, 44(38), 7179-7181 (2003)
Yuuka Masuyama et al.
Drug metabolism and pharmacokinetics, 35(3), 274-280 (2020-04-20)
Flavin containing monooxygenases (FMOs) represent one of the predominant types of phase I drug metabolizing enzymes (DMEs), and thus play an important role in the metabolism of xeno- and endobiotics for the generation of their corresponding oxides. These oxides often
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.