343609
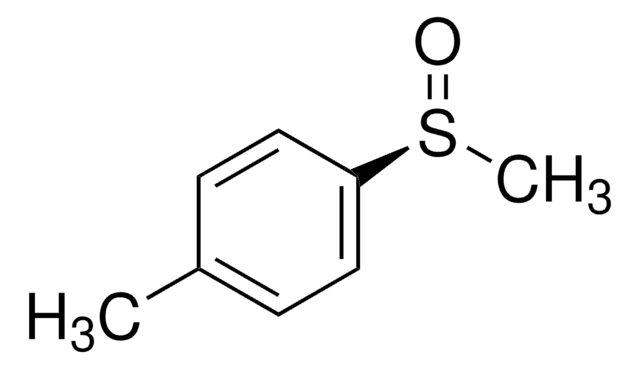
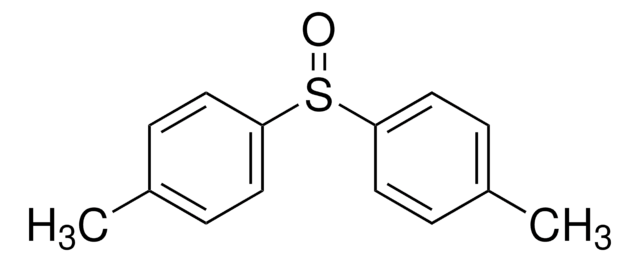
(R)-(+)-Methyl p-tolyl sulfoxide
99%
동의어(들):
(+)-(R)-Methyl 4-tolyl sulfoxide, (+)-Methyl p-tolyl sulfoxide, (R)-4-Methylphenyl methyl sulfoxide, 1-Methyl-4-[(R)-methylsulfinyl]benzene
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH3C6H4S(O)CH3
CAS Number:
Molecular Weight:
154.23
Beilstein:
2040677
MDL number:
UNSPSC 코드:
12191600
PubChem Substance ID:
NACRES:
NA.22
분석:
99%
추천 제품
분석
99%
광학 활성
[α]20/D +145°, c = 2 in acetone
광학 순도
ee: 99% (HPLC)
mp
73-75 °C (lit.)
작용기
sulfoxide
SMILES string
Cc1ccc(cc1)S(C)=O
InChI
1S/C8H10OS/c1-7-3-5-8(6-4-7)10(2)9/h3-6H,1-2H3/t10-/m1/s1
InChI key
FEVALTJSQBFLEU-SNVBAGLBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(R)-(+)-Methyl p-tolyl sulfoxide may be used to prepare (R)-(+)-methyl 3,5-dimethoxy-6-[8-oxo-9-(p-tolylsulfinyl) nonyl] benzoate, an intermediate for (R)-lasiodiplodin synthesis. Its anions undergo addition reaction with nitrones to form optically active a-substituted N-hydroxylamines. (R)-(+)-Methyl p-tolyl sulfoxide also reacts with O-mesitylsulfonylhydroxylamine (MSH) to form (-)-(R)-S-methyl-S-p-tolylsulfoximine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Chemistry of sulfoxides and related compounds. XLIX. Synthesis of optically active sulfoximines from optically active sulfoxides.
Johnson CR, et al.
The Journal of Organic Chemistry, 39(16), 2458-2459 (1974)
Asymmetric synthesis of orsellinic acid type macrolides: The example of lasiodiplodin.
Solladie G, et al.
Tetrahedron Asymmetry, 1(3), 187-198 (1990)
The reaction of nitrones with (R)-(+)-methyl p-tolyl sulfoxide anion; asymmetric synthesis of optically active secondary amines.
Murahashi S-I, et al.
Tetrahedron Letters, 34(16), 2645-2648 (1993)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.