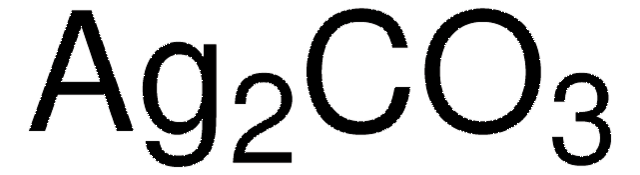483346
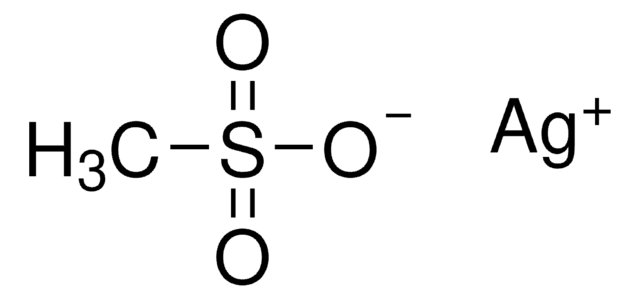
Silver trifluoromethanesulfonate
≥99.95% trace metals basis
동의어(들):
AgOTf, Silver triflate, Trifluoromethanesulfonic acid silver salt
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
CF3SO3Ag
CAS Number:
Molecular Weight:
256.94
Beilstein:
3598402
EC Number:
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
- Silver trifluoromethanesulfonate (AgOTf ) is a reactive triflating agent, which converts alkyl, acyl and sulfonyl halides to corresponding triflate species.
- It is a highly suitable electrophile to initiate acetylenic oxy-Cope rearrangement of substituted 5-hexen-1-yn-3-ols to synthesize corresponding α,δ-diethylenic aldehydes.
- It can also be used in the diastereoselective cyclization of amino ketenes where the diastereoselectivity depends on Ag(I) concentration.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Perfluoroalkanesulfonic esters: methods of preparation and applications in organic chemistry.
Stang P J, et al.
Synthesis, 1982(02), 85-126 (1982)
Asymmetric synthesis via electrophile-mediated cyclisations.
Fox D N and Gallagher T
Tetrahedron, 46(13-14), 4697-4710 (1990)
Silver mediated acetylenic oxy cope rearrangement.
Bluthe N, et al.
Tetrahedron, 42(5), 1333-1344 (1986)
Trifluoromethanesulfonic?Carboxylic Anhydrides, Highly Active Acylating Agents.
Effenberger F and Epple G
Angewandte Chemie (International Edition in English), 11(4), 299-300 (1972)
Dennis M Whitfield
Carbohydrate research, 356, 180-190 (2012-04-25)
The Transition State (TS) for any chemical glycosylation reaction is not known with certainty. Both experimental and computational approaches have been limited due to the complexity of the problem. This work describes a preliminary computational ionization approach using density functional
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.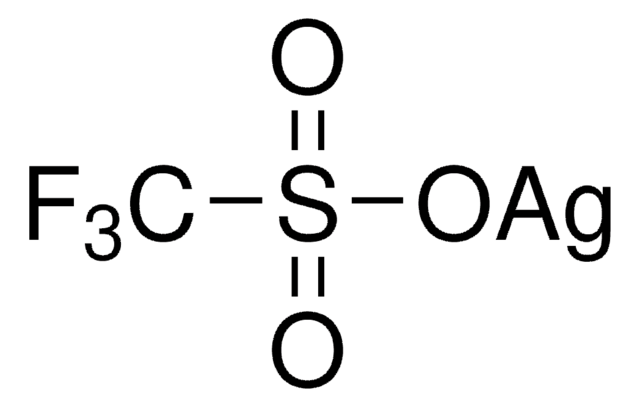









![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)