추천 제품
Quality Level
분석
≥95%
mp
256-260 °C (lit.)
SMILES string
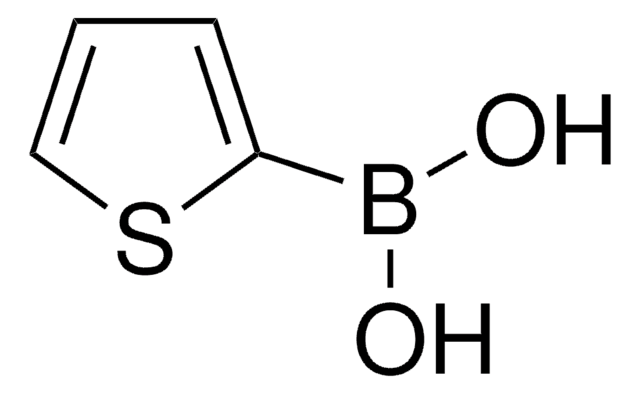
OB(O)c1cc2ccccc2s1
InChI
1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H
InChI key
YNCYPMUJDDXIRH-UHFFFAOYSA-N
애플리케이션
Reactant involved in:
- PDE4 inhibitors
- Chemoselective modification of oncolytic adenovirus
- Synthesis of phosphorescent sensor for quantification of copper(II) ion
- UV promoted phenanthridine syntheses
- Preparation of CYP11B1 inhibitors for treatment of cortisol dependent diseases
- Suzuki-Miyaura cross-coupling reactions
기타 정보
Contains varying amounts of anhydride
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
S A Adediran et al.
Archives of biochemistry and biophysics, 614, 65-71 (2017-01-01)
O-Aryloxycarbonyl hydroxamates have previously been shown to covalently inactivate serine/amine amidohydrolases such as class C β-lactamases and a N-terminal hydrolase, the proteasome. We report here reactions between O-aryloxycarbonyl hydroxamates and another N-terminal hydrolase, penicillin acylase. O-Aryloxycarbonyl hydroxamates, as non-symmetric carbonates
Ju Hyoung Jo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(70), 16733-16754 (2020-07-07)
Herein, we report the synthesis, and photochemical and -physical properties, as well as the catalytic performance, of a series of heteroleptic IrIII photosensitizers (IrPSs), [Ir(C^N)2 (N^NAryl )]+ , possessing ancillary ligands that are varied with aryl-substituents on bipyridyl unit [C^N=(2-pyridyl)benzo[b]thiophen-3-yl
Hyomin Jin et al.
Dalton transactions (Cambridge, England : 2003), 48(4), 1467-1476 (2019-01-12)
2-Phenylpyridine- and 2-(benzo[b]thiophen-2-yl)pyridine-based (ppy- and btp-based) o-carboranyl (Car1 and Car2) and their B(CH3)2-C∧N-chelated (Car1B and Car2B) compounds were prepared and fully characterised by multinuclear NMR spectroscopy and elemental analysis. The solid-state structure of Car2B was determined by single-crystal X-ray diffraction
Ramona Iseppi et al.
Microbial drug resistance (Larchmont, N.Y.), 24(8), 1156-1164 (2018-02-17)
We investigated the occurrence of extended-spectrum β-lactamase (ESBL), AmpC, and carbapenemase-producing Gram-negative bacteria isolated from 160 samples of fresh vegetables (n = 80) and ready-to-eat (RTE) prepacked salads (n = 80). Phenotypic and genotypic analyses were carried out on the isolates in terms of
Alberto Venturelli et al.
Journal of medicinal chemistry, 50(23), 5644-5654 (2007-10-25)
Benzo[b]thiophene-2-ylboronic acid, 1, is a 27 nM inhibitor of the class C beta-lactamase AmpC and potentiates the activity of beta-lactam antibiotics in bacteria that express this and related enzymes. As is often true, the potency of compound 1 against the
문서
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




![Benzo[b]thien-3-ylboronic acid ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/136/961/9ddc053e-3519-47d3-be03-95715d131635/640/9ddc053e-3519-47d3-be03-95715d131635.png)