565814
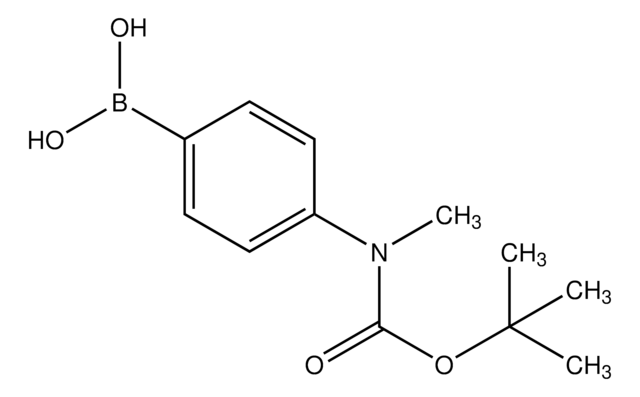
4-(N-Boc-amino)phenylboronic acid
≥95.0%
동의어(들):
4-(Boc-amino)phenylboronic acid, 4-[(tert-Butoxycarbonyl)amino]benzeneboronic acid, C-(1,1-Dimethylethyl) N-(4-boronophenyl)carbamate, [4-(N-Bocamino)phenyl]boronic acid, [4-(tert-Butoxycarbonylamino)phenyl]boronic acid, [4-[[[(1,1-Dimethylethyl)oxy]carbonyl]amino]phenyl]boronic acid
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
(CH3)3CO2CNHC6H4B(OH)2
CAS Number:
Molecular Weight:
237.06
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95.0%
형태
solid
mp
199-204 °C (dec.) (lit.)
작용기
amine
SMILES string
CC(C)(C)OC(=O)Nc1ccc(cc1)B(O)O
InChI
1S/C11H16BNO4/c1-11(2,3)17-10(14)13-9-6-4-8(5-7-9)12(15)16/h4-7,15-16H,1-3H3,(H,13,14)
InChI key
UBVOLHQIEQVXGM-UHFFFAOYSA-N
애플리케이션
Boronic acid used in a study of the rhodium-catalyzed desymmetrization of a meso-cyclic allylic dicarbonate via SN2′ substitution.
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Frederic Menard et al.
Organic letters, 8(20), 4569-4572 (2006-09-22)
An enantio-, regio-, and diastereoselective rhodium(I)-catalyzed desymmetrization of a meso-cyclic allylic dicarbonate with organoboronic acid nucleophiles is described. The rhodium(I) catalyst formed in situ from [Rh(cod)OH]2 and Xyl-P-PHOS allowed the S(N)2' allylic substitution product to be obtained with a range
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.