659878
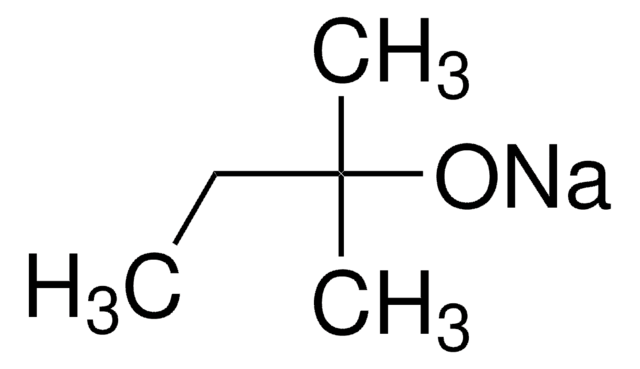
Potassium tert-butoxide
sublimed grade, 99.99% trace metals basis
동의어(들):
Potassium tert-butylate, Potassium t-butoxide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3COK
CAS Number:
Molecular Weight:
112.21
Beilstein:
3556712
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
일반 설명
Potassium tert-butoxide is a strong alkoxide base, it can deprotonate carbon and other Brφnsted acids. It is a relatively poor nucleophile.
Total impurities: may contain up to 5,000 ppm Sodium
애플리케이션
Mizoroki-Heck-Type reactions are mediated by potassium tert-butoxide. potassium tert-butoxide can commence the anionic polymerization of carbazolyl-substituted oxiranes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 2 - Skin Corr. 1A
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Anionic polymerization of carbazolyl-substituted oxiranes initiated by potassium alkalide, potassium tert-butoxide and potassium hydride.
Buika G, et al.
Macromolecular Chemistry and Physics, 196(4), 1287-1293 (1995)
Einav Amit et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(57), 13046-13052 (2020-04-29)
N-heterocyclic carbenes (NHCs) have emerged as a unique molecular platform for the formation of self-assembled monolayers (SAMs) on various surfaces. However, active carbene formation requires deprotonation of imidazolium salt precursors, which is mostly facilitated by exposure of the salt to
H Takigami et al.
Water science and technology : a journal of the International Association on Water Pollution Research, 53(11), 43-50 (2006-07-26)
Capacitor oil samples (PCBs > 90%wt) were treated in a bench scale experiment to investigate the destruction of PCBs during chemical destruction processes (a catalytic hydrodechlorination treatment with palladium carbon and additional treatment with potassium tert-butyloxide). Using those results, this
Aurélie Mallinger et al.
The Journal of organic chemistry, 74(3), 1124-1129 (2008-12-24)
3-Aryltetronic acids were prepared in one step by treatment of a mixture of methyl arylacetates and methyl hydroxyacetates with potassium tert-butoxide, via tandem transesterification/Dieckmann condensation. Several mushroom or lichen pigments, vulpinic acids, were synthesized from 3-(4-methoxyphenyl)tetronic acid in three steps
Scott E Denmark et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(19), 4954-4963 (2006-05-04)
This paper chronicles the conceptual development, proof of principle experiments, and recent advances in the palladium-catalyzed cross-coupling reactions of the conjugate bases of organosilanols. The discovery that led to the design and refinement of this process represents a classical illustration
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.