추천 제품
Quality Level
분석
≥95%
양식
solid
mp
100-104 °C
작용기
phosphine
SMILES string
CP(c1nc2ccccc2nc1P(C)C(C)(C)C)C(C)(C)C
InChI
1S/C18H28N2P2/c1-17(2,3)21(7)15-16(22(8)18(4,5)6)20-14-12-10-9-11-13(14)19-15/h9-12H,1-8H3/t21-,22-/m0/s1
InChI key
DRZBLHZZDMCPGX-VXKWHMMOSA-N
일반 설명
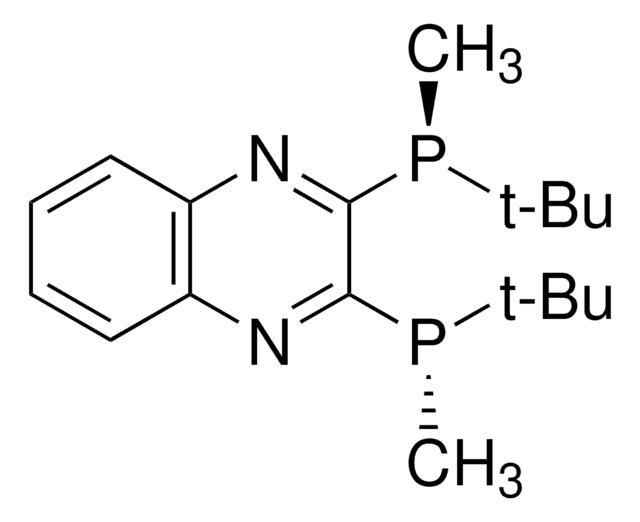
(R,R)-(-)-2,3-Bis(tert-butylmethylphosphino)quinoxaline is an air-stable C2-symmetric P-stereogenic phosphine ligand.
애플리케이션
Efficient ligand exhibiting high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed 1,4-addition of arylboronic acids to alkylative ring opening to asymmetric hydrogenation.
특징 및 장점
Advantages of the QuinoxP* Ligands:
- It is not oxidized nor epimerized at ambient conditions in air
- Enantioselectivities are outstanding for various reaction paradigms
- Hydrogenations proceed under mild reaction conditions
- Low catalyst loadings yield high TONs
문헌인용
Fang P and Hou XL. "Asymmetric Copper-Catalyzed Propargylic Substitution Reaction of Propargylic Acetates with Enamines." Organic letters 11.20 (2009): 4612-4615.
Imamoto T, et al. "Highly enantioselective hydrosilylation of simple ketones catalyzed by rhodium complexes of P-chiral diphosphine ligands bearing tert-butylmethylphosphino groups." Tetrahedron: Asymmetry 17(4) (2006): 560-565.
Yahav-Levi A, et al. "Aryl-bromide reductive elimination from an isolated Pt (IV) complex." Chemical Communications 46(19) (2010): 3324-3326.
법적 정보
QuinoxP is a registered trademark of Nippon Chemical Industry Co., Ltd.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Highly enantioselective hydrosilylation of simple ketones catalyzed by rhodium complexes of P-chiral diphosphine ligands bearing tert-butylmethylphosphino groups.
Imamoto T, et al.
Tetrahedron Asymmetry, 17(4), 560-565 (2006)
A rational pre-catalyst design for bis-phosphine mono-oxide palladium catalyzed reactions.
Ji Y, et al.
Chemical Science (2017)
Asymmetric Copper-Catalyzed Propargylic Substitution Reaction of Propargylic Acetates with Enamines.
Fang P and Hou XL.
Organic Letters, 11.20, 4612-4615 (2009)
Three-Hindered Quadrant Phosphine Ligands with an Aromatic Ring Backbone for the Rhodium-Catalyzed Asymmetric Hydrogenation of Functionalized Alkenes.
Zhang Z, et al.
The Journal of Organic Chemistry, 77(8), 4184-4188 (2012)
Aryl-bromide reductive elimination from an isolated Pt (IV) complex.
Yahav-Levi A, et al.
Chemical Communications (Cambridge, England), 46(19), 3324-3326 (2010)
문서
QuinoxP*: Air-Stable and Highly Efficient and Productive Chiral Ligands
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)
![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)