추천 제품
Quality Level
분석
95%
양식
liquid
광학 활성
[α]20/D -48.0°, c = 1 in chloroform
refractive index
n20/D 1.544
density
1.175 g/mL at 25 °C
작용기
chloro
hydroxyl
SMILES string
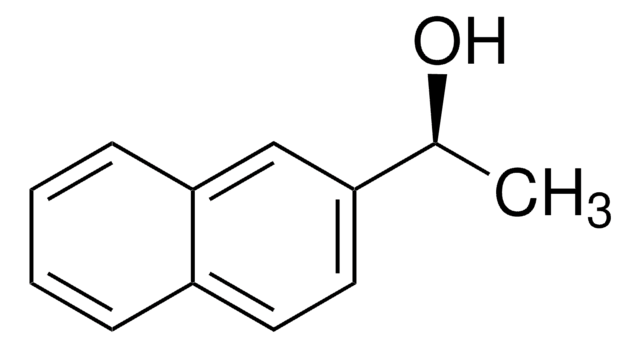
C[C@H](O)c1ccc(Cl)cc1
InChI
1S/C8H9ClO/c1-6(10)7-2-4-8(9)5-3-7/h2-6,10H,1H3/t6-/m0/s1
InChI key
MVOSNPUNXINWAD-LURJTMIESA-N
애플리케이션
(S)-4-Chloro-α-methylbenzyl alcohol can be used as a chiral building block in:
- The enantioselective synthesis of (-)-(S,S)-clemastine.
- The enantioselective and geometrically defined synthesis of chloro methylbenzyl pinacol boronic ester via lithiation−borylation methodology.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
>230.0 °F
Flash Point (°C)
> 110 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
Stereospecific conversion of alcohols into pinacol boronic esters using lithiation-borylation methodology with pinacolborane
Roesner S, et al.
Chemical Communications (Cambridge, England), 50(31), 4053-4055 (2014)
Anne M Fournier et al.
Organic letters, 12(10), 2222-2225 (2010-04-22)
The first enantioselective synthesis of the antihistamine agent clemastine, as its (S,S)-stereoisomer, has been achieved by ether formation between a proline-derived chloroethylpyrrolidine and an enantiomerically enriched tertiary alcohol. The tertiary alcohol was formed from the carbamate derivative of alpha-methyl-p-chlorobenzyl alcohol
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.