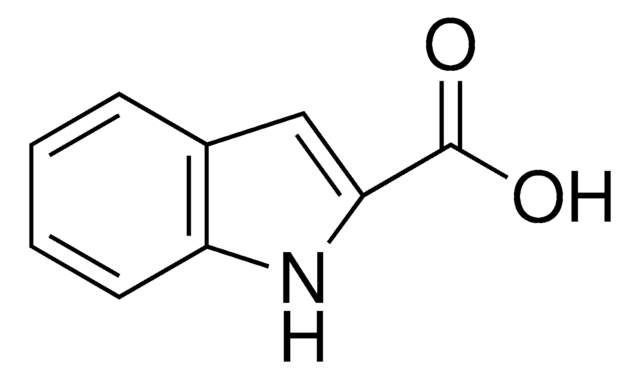
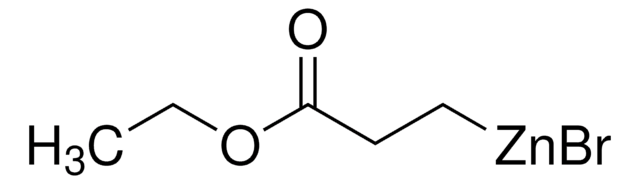
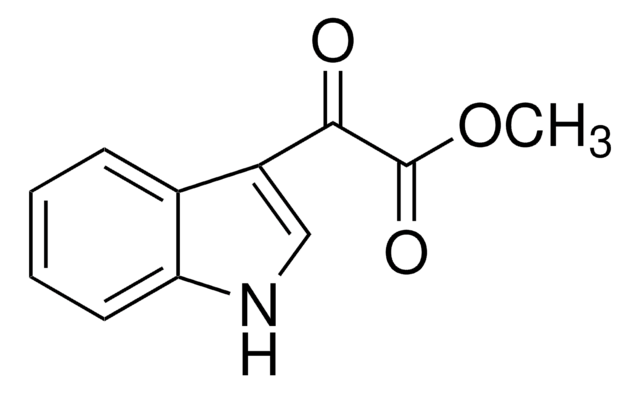
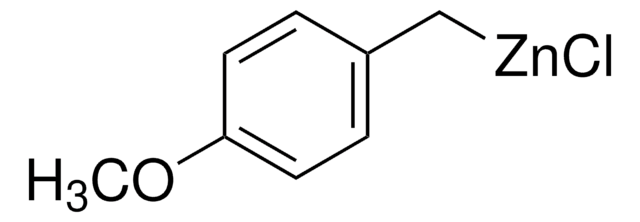
추천 제품
양식
liquid
Quality Level
반응 적합성
reaction type: C-C Bond Formation
농도
0.6 M in THF
density
0.992 g/mL at 25 °C
작용기
ether
저장 온도
2-8°C
SMILES string
Br[Zn]CC1OCCO1
InChI
1S/C4H7O2.BrH.Zn/c1-4-5-2-3-6-4;;/h4H,1-3H2;1H;/q;;+1/p-1
InChI key
LOJRLQRGBKTHDB-UHFFFAOYSA-M
일반 설명
(1,3-Dioxolan-2-ylmethyl)zinc bromide is an organozinc compound used as a reagent in Negishi cross-coupling reaction to prepare aryl or heteroaryl scaffolds via C-C bond formation.
애플리케이션
(1,3-Dioxolan-2-ylmethyl)zinc bromide can be used as a reagent in the allylic alkylation reactions in the presence of iridium catalyst.
법적 정보
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-22.0 °F
Flash Point (°C)
-30 °C
Iridium-Catalyzed Enantioselective Allylic Alkylation with Functionalized Organozinc Bromides
Hamilton JY, et al.
Angewandte Chemie (International ed. in English), 54(26), 7644-7647 (2015)
Synthesis of 8-C-substituted 2, 6-diaminopurine acyclic nucleoside phosphonates by Negishi cross-coupling
Sedlavcek O, et al.
Collection of Czechoslovak Chemical Communications, 10(26), 449-450 (2015)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![2-(1,3-Dioxolan-2-yl)]ethyl]zinc bromide solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/208/700/ec5d0440-e703-4c3f-80f9-95d43bbef39b/640/ec5d0440-e703-4c3f-80f9-95d43bbef39b.png)