79421
Phosphazene base P4-t-Bu solution
~0.8 M in hexane
동의어(들):
1-tert-Butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)-phosphoranylidenamino]-2λ5,4λ5-catenadi(phosphazene)
About This Item
추천 제품
양식
liquid
Quality Level
농도
~0.8 M in hexane
density
0.850-0.875 g/mL at 20 °C
작용기
amine
SMILES string
CN(C)P(=NP(=NC(C)(C)C)(N=P(N(C)C)(N(C)C)N(C)C)N=P(N(C)C)(N(C)C)N(C)C)(N(C)C)N(C)C
InChI
1S/C22H63N13P4/c1-22(2,3)23-36(24-37(27(4)5,28(6)7)29(8)9,25-38(30(10)11,31(12)13)32(14)15)26-39(33(16)17,34(18)19)35(20)21/h1-21H3
InChI key
NSRBCQCXZAYQHF-UHFFFAOYSA-N
주의사항
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 1 Inhalation - STOT SE 3
표적 기관
Central nervous system, Nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-14.8 °F - closed cup
Flash Point (°C)
-26 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
문서
Phosphazene base reagents are available as monomeric (P1 and BEMP), dimeric (P2), and tetrameric (P4) bases with different side chains to control their sterical hindrance.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.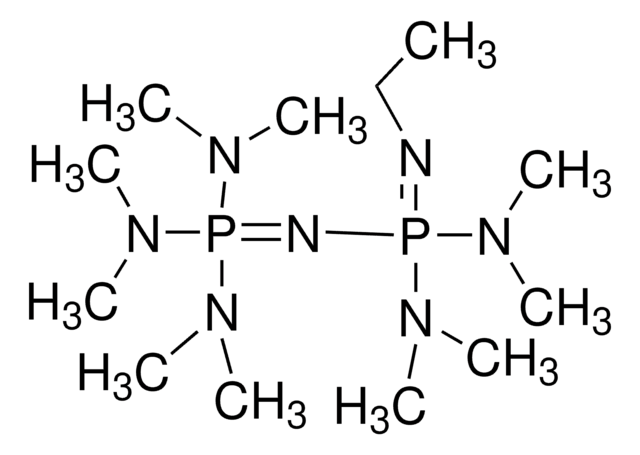


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






