추천 제품
양식
liquid
반응 적합성
reaction type: C-H Activation
reagent type: catalyst
reagent type: oxidant
refractive index
n/D 1.444
density
1.361 g/mL
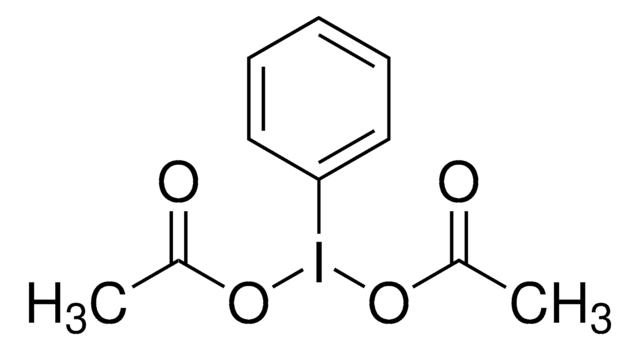
InChI
1S/C10H11IO4/c1-8(12)14-11(15-9(2)13)10-6-4-3-5-7-10/h3-7H,1-2H3
InChI key
ZBIKORITPGTTGI-UHFFFAOYSA-N
애플리케이션
Stoichiometric oxidant in the TEMPO oxidation of nerol to neral. Oxidant employed in the rhodium-catalyzed aziridination of olefins with sulfamate esters.
Unactivated sp3 C-H bonds of both oxime and pyridine substrates undergo highly regio- and chemoselective Pd(II)-catalyzed oxygenation with PhI(OAc)2 as a stoichiometric oxidant.
Used in the room temperature Pd-catalyzed 2-arylation of indoles
Useful reagent for the synthesis of a wide variety of heterocyclic compounds.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Tetrahedron, 62, 11331-11331 (2006)
Renhua Fan et al.
Organic & biomolecular chemistry, 6(24), 4615-4621 (2008-11-29)
An efficient one-pot oxidative decarboxylation-Friedel-Crafts reaction of acyclic alpha-amino acid derivatives with electron-rich aromatic compounds is reported. The reaction is activated by the combination of iodobenzene diacetate, iodine and iron dust, resulting in a mild and simple reaction system. The
Jinbo Huang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(44), 13964-13967 (2012-09-22)
A metal-free synthesis of diversified benzimidazoles from N-arylamidines through a phenyliodine(III) diacetate (PIDA) promoted intramolecular direct C(sp(2))-H imidation has been developed. The reaction proceeds smoothly at 0 °C or ambient temperature to provide the desired products in good to excellent
Jayasree Seayad et al.
Organic letters, 12(7), 1412-1415 (2010-03-13)
Copper(I) or -(II) salts with weakly coordinating anions catalyze the diacetoxylation of olefins efficiently in the presence of PhI(OAc)(2) as the oxidant under mild conditions. The reaction is effective for aryl, aryl alkyl, as well as aliphatic terminal and internal
Hai Yi et al.
Chemical communications (Cambridge, England), 46(37), 6941-6943 (2010-08-24)
3-Aryl-4-unsubstituted-6-CF(3)-pyridin-2-ones have been efficiently synthesized from readily available 4-aryl-3-carbamoyl-6-CF(3)-pyridin-2(1H)-ones by treatment with PhI(OAc)(2) in the presence of NaOH.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.