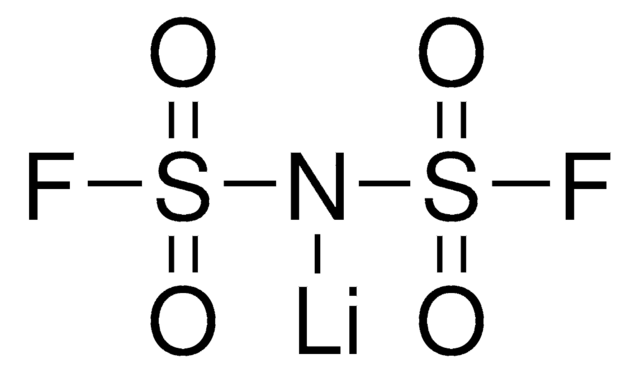919977
Lithium bis(trifluoromethanesulfonyl)imide
anhydrous, 99.99% trace metals basis
동의어(들):
Bis(trifluoromethane)sulfonimide lithium salt, LiNTf2, LiTFSI, LiTf2N, Bis(trifluoromethylsulfonyl)amine lithium salt, Lithium bistrifluoromethanesulfonimidate
About This Item
추천 제품
Grade
anhydrous
Quality Level
분석
99.99% trace metals basis
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
234-238 °C (lit.)
응용 분야
battery manufacturing
환경친화적 대안 카테고리
SMILES string
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
InChI key
QSZMZKBZAYQGRS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- An additive in the development of dual-functional separator coating materials. These materials are based on covalent organic frameworks (COFs) and are specifically designed for use in high-performance lithium-selenium sulfide batteries. The Li-SeS2 battery achieved outstanding performance in terms of energy storage and stability. It exhibited a specific capacity of 844.6 mA h g-1 at 0.5C and a SeS2 loading of 2 mg cm-2.
- As an additive in the electrolyte formulation along with polyethylene oxide for the development of solid-state lithium batteries. LiTFSI enhance the ionic conductivity of the PEO-based electrolyte, which is essential for the efficient transport of lithium ions.
- As a key component in the development of a PEO/LiTFSI-coated polypropylene membrane. This membrane is designed for high-loading lithium–sulfur batteries to enhance battery performance, improve capacity, and extend cycle life.
- As a component in the electrolyte system along with TEMPOL derivatives. The incorporation of LiTFSI in the electrolyte system enhances the stability and achieves an efficiency of 6.16% in solid-state fiber dye-sensitized solar cells.
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
표적 기관
Nervous system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.