추천 제품
양식
powder
Quality Level
반응 적합성
reagent type: oxidant
SMILES string
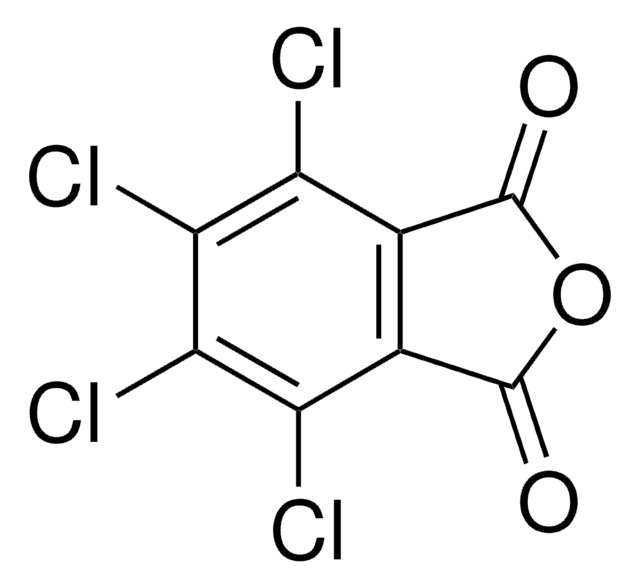
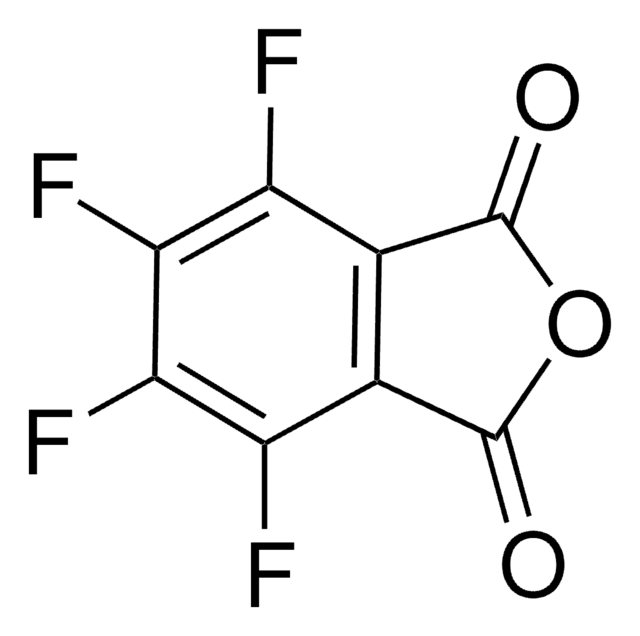
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI key
UTRBHXSKVVPTLY-UHFFFAOYSA-N
일반 설명
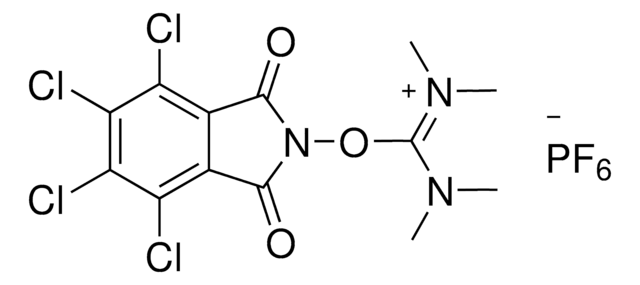
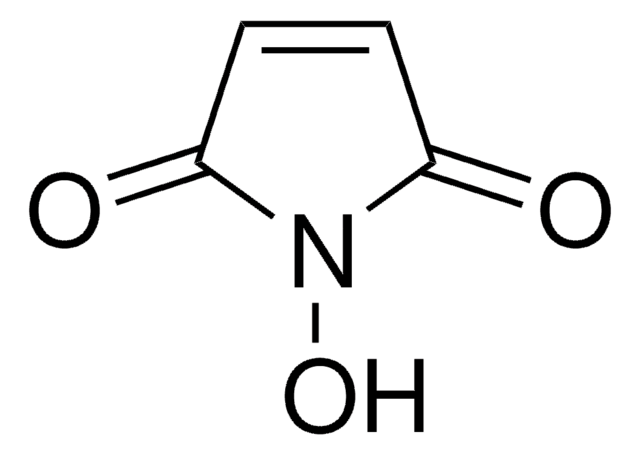
N-Hydroxytetrachlorophthalimide (TCNHPI) is an aryl-tetrahalogenated N-hydroxyphthalimide derivative. In combination with 1,4-diamino-2,3-dichloroanthraquinone (DADCAQ), it forms an efficient catalytic system for the oxidation of aromatic hydrocarbons using molecular oxygen under metal-free and aerobic conditions.
애플리케이션
New reagent enabling the electrochemical allylic C-H oxidation reaction developed by the Baran group. This method provides a scalable and sustainable alternative to current strategies based on toxic reagents or precious metals.
기타 정보
Electrochemical Allylic C–H Oxidation with N-Hydroxytetrachlorophthalimide (TCNHPI)
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Tian Qin et al.
Science (New York, N.Y.), 352(6287), 801-805 (2016-04-23)
Alkyl carboxylic acids are ubiquitous in all facets of chemical science, from natural products to polymers, and represent an ideal starting material with which to forge new connections. This study demonstrates how the same activating principles used for decades to
Jie Wang et al.
Angewandte Chemie (International ed. in English), 55(33), 9676-9679 (2016-07-07)
A transformation analogous in simplicity and functional group tolerance to the venerable Suzuki cross-coupling between alkyl-carboxylic acids and boronic acids is described. This Ni-catalyzed reaction relies upon the activation of alkyl carboxylic acids as their redox-active ester derivatives, specifically N-hydroxy-tetrachlorophthalimide
Josep Cornella et al.
Journal of the American Chemical Society, 138(7), 2174-2177 (2016-02-03)
A new transformation is presented that enables chemists to couple simple alkyl carboxylic acids with aryl zinc reagents under Ni-catalysis. The success of this reaction hinges on the unique use of redox-active esters that allow one to employ such derivatives
Efficient metal-free aerobic oxidation of aromatic hydrocarbons utilizing aryl-tetrahalogenated N-hydroxyphthalimides and 1,4-diamino-2,3-dichloroanthraquinone.
Zhang Q, et al.
Journal of Chemical Technology and Biotechnology, 83(10), 1364-1369 (2008)
문서
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
관련 콘텐츠
Baran Group offers zinc-based reagents for versatile transformations in organic synthesis.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.