추천 제품
Quality Level
분석
98%
형태
powder
광학 활성
[α]20/D +59.7°, c = 2 in H2O
색상
white to off-white
mp
107 - 110 °C ((225 - 230 °F))
107-110 °C (lit.)
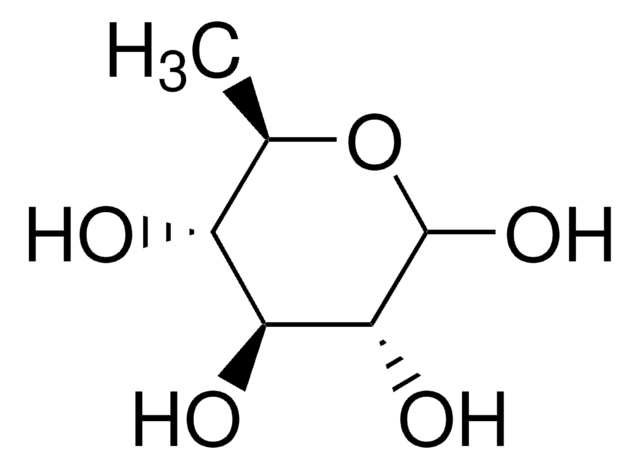
SMILES string
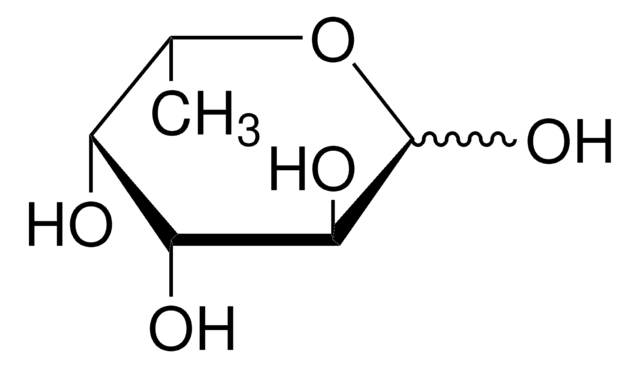
OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI
1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
InChI key
PMMURAAUARKVCB-DUVQVXGLSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Deoxy-D-galactose is a glucose analog that shows a wide range of biological activities such as inhibition of glycolysis and thereby tumor growth, interference with the biosynthetic processing of glycoproteins, antiviral activity, and hepatotoxicity. It is being extensively studied as trapping agents for phosphate and uridylate in mammalian cells due to its ability to interfere in the phosphate and nucleotide metabolism.
애플리케이션
- FUT1-mediated terminal fucosylation acts as a new target to attenuate renal fibrosis.: This research investigates the role of 2-deoxy-D-galactose in modulating terminal fucosylation processes, revealing potential therapeutic pathways for treating renal fibrosis and enhancing the understanding of kidney disease mechanisms (Luo et al., 2023).
- 2-D-gal Targets Terminal Fucosylation to Inhibit T-cell Response in a Mouse Skin Transplant Model.: Highlights the immunomodulatory potential of 2-deoxy-D-galactose in transplant medicine, showing how it can inhibit T-cell responses and contribute to the success of skin grafts, pointing towards new immunosuppressive treatments (Mao et al., 2023).
- Inhibition of Aberrant α(1,2)-Fucosylation at Ocular Surface Ameliorates Dry Eye Disease.: Explores the therapeutic effects of 2-deoxy-D-galactose in treating dry eye disease by modulating specific fucosylation pathways, potentially opening new avenues for ocular surface treatment strategies (Yoon et al., 2021).
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Yaqin Wang et al.
Food chemistry, 285, 221-230 (2019-02-25)
The effect of dextran produced in situ by Weissella confusa on the structure and nutrition quality of whole grain pearl millet bread containing 50% of wheat flour was investigated. NMR spectroscopy analysis indicated that the dextran formed by the strain
S Bullock et al.
Journal of neurochemistry, 54(1), 135-142 (1990-01-01)
The interaction of the amnesic agent 2-deoxygalactose with fucose incorporation into glycoproteins in day-old chick forebrain has been studied with the aim of identifying glycoproteins whose synthesis is modified during memory formation. 2-Deoxygalactose inhibited total exogenous [14C]fucose incorporation into the
Impairment of glycoprotein fucosylation in rat hippocampus and the consequences on memory formation.
R Jork et al.
Pharmacology, biochemistry, and behavior, 25(6), 1137-1144 (1986-12-01)
The intraventricular injection of 2-deoxy-D-galactose led to a dose- and time-dependent decrease in the fucosylation of hippocampal glycoproteins in rats whereas the incorporation of 3H-N-acetyl-glucosamine was not influenced. This effect is not related to an interference with fucose activating or
Matthew Z Anderson et al.
Nature communications, 10(1), 4388-4388 (2019-09-29)
Meiosis is a conserved tenet of sexual reproduction in eukaryotes, yet this program is seemingly absent from many extant species. In the human fungal pathogen Candida albicans, mating of diploid cells generates tetraploid products that return to the diploid state
Yunrong Chai et al.
mBio, 3(4), e00184-e00112 (2012-08-16)
Galactose is a common monosaccharide that can be utilized by all living organisms via the activities of three main enzymes that make up the Leloir pathway: GalK, GalT, and GalE. In Bacillus subtilis, the absence of GalE causes sensitivity to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.