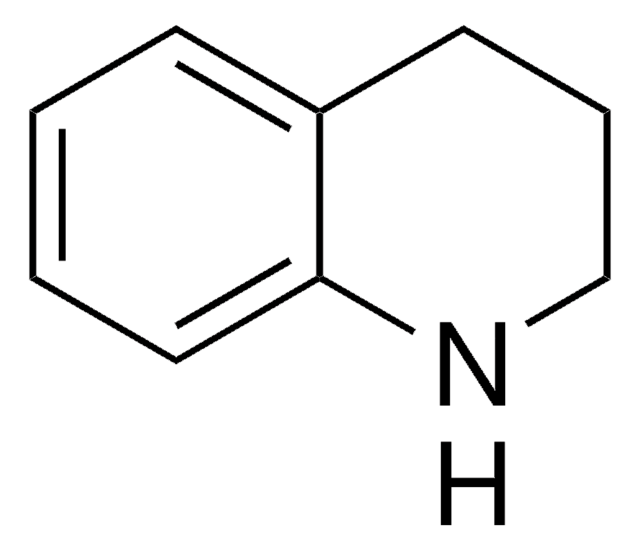
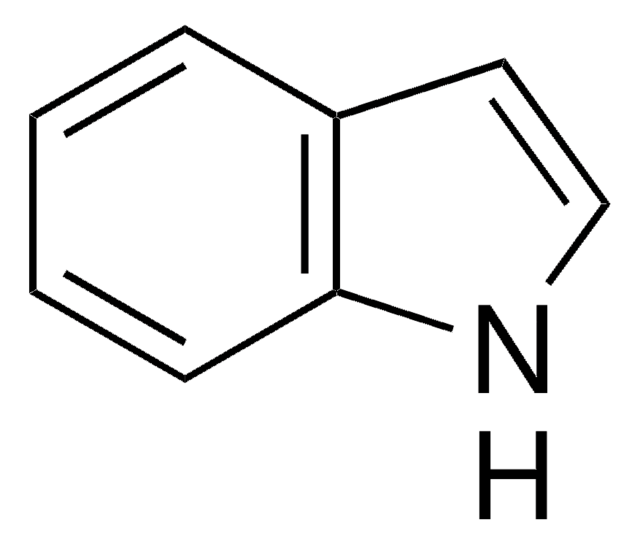
추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
양식
liquid
refractive index
n20/D 1.592 (lit.)
bp
220-221 °C (lit.)
density
1.063 g/mL at 25 °C (lit.)
SMILES string
C1Cc2ccccc2N1
InChI
1S/C8H9N/c1-2-4-8-7(3-1)5-6-9-8/h1-4,9H,5-6H2
InChI key
LPAGFVYQRIESJQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Reactant for preparation of:
- Inhibitors of NOD1-Induced Nuclear Factor-κB Activation
- Sphingosine-1-phosphate 4(S1P4) receptor antagonists
- Cytotoxic cell cycle inhibitors
- 2-Aminopyridines
- PET agent for imaging of protein kinase C (PKC)
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- α4β2-Nicotinic acetylcholine receptor-selective partial agonists
- mGlu4 positive allosteric modulators
- Bacterial biofilm inhibitors
- Serotonin 5-HT6 receptor antagonists
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
199.4 °F - closed cup
Flash Point (°C)
93 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Matej Baláž et al.
Molecules (Basel, Switzerland), 24(18) (2019-09-22)
Performing solution-phase oximation reactions with hydroxylamine hydrochloride (NH2OH·HCl) carries significant risk, especially in aqueous solutions. In the present study, four N-substituted indole-3-carboxaldehyde oximes were prepared from the corresponding aldehydes by solvent-free reaction with NH2OH·HCl and a base (NaOH or Na2CO3)
Toshiharu Noji et al.
Organic letters, 15(8), 1946-1949 (2013-04-04)
A benzyne-mediated synthesis of substituted indolines and carbazoles was developed. The reaction includes generation of benzyne using Mg(TMP)2·2LiCl as a base, cyclization, and trapping the resulting organomagnesium intermediate with an electrophile to provide a series of substituted indolines and carbazoles
François Brucelle et al.
Organic letters, 14(12), 3048-3051 (2012-06-01)
A simple approach to prepare indolines and benzopyrrolizidinones from ortho-azidoallylbenzenes via a tandem radical addition/cyclization is described. The use of triethylborane to initiate and sustain the process provides the best results. Indolines are easily converted into the corresponding indoles by
Fengtao Zhou et al.
Journal of the American Chemical Society, 134(35), 14326-14329 (2012-08-24)
The first highly enantioselective copper-catalyzed intramolecular Ullmann C-N coupling reaction has been developed. The asymmetric desymmetrization of 1,3-bis(2-iodoaryl)propan-2-amines catalyzed by CuI/(R)-BINOL-derived ligands led to the enantioselective formation of indolines in high yields and excellent enantiomeric excesses. This method was also
Jan Michael Schuller et al.
Journal of molecular biology, 422(1), 87-99 (2012-06-12)
Fungal indole prenyltransferases (PTs) typically act on specific substrates, and they are able to prenylate their target compounds with remarkably high regio- and stereoselectivity. Similar to several indole PTs characterized to date, the cyclic dipeptide N-prenyltransferase (CdpNPT) is able to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.