T49409
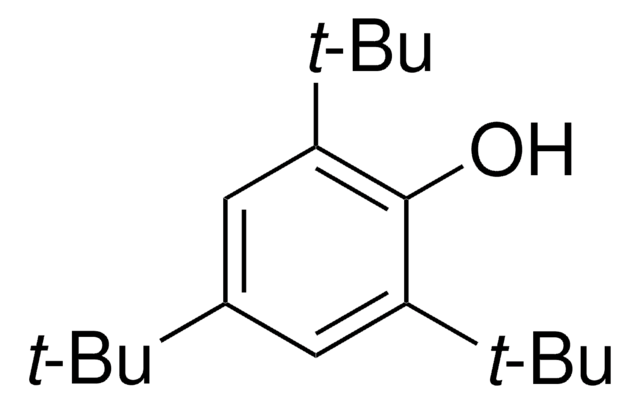
2,4,6-Tri-tert-butylphenol
98%
동의어(들):
2,4,6-Tri-t-butylphenol, 2,4,6-Tri-tert-butyl-1-hydroxybenzene, 2,4,6-Tris(1,1-dimethylethyl)phenol, 2,4,6-Tris(tert-butyl)phenol, 2,4,6-Tritert-butylphenol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
[(CH3)3C]3C6H2OH
CAS Number:
Molecular Weight:
262.43
Beilstein:
1913256
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B - Skin Sens. 1B - STOT RE 2
표적 기관
Liver
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Christian R Goldsmith et al.
Journal of the American Chemical Society, 124(1), 83-96 (2002-01-05)
Lipoxygenases are mononuclear non-heme iron enzymes that regio- and stereospecifcally convert 1,4-pentadiene subunit-containing fatty acids into alkyl peroxides. The rate-determining step is generally accepted to be hydrogen atom abstraction from the pentadiene subunit of the substrate by an active ferric
S Nemoto et al.
Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 42(6), 359-366 (2002-03-06)
An analytical method has been developed for the determination of 2,4,6-tri-tert-butylphenol (TTBP) in foods. TTBP was determined by GC/MS (SIM) after extraction from food samples using a steam distillation technique. The developed method was able to determine simultaneously 2,4-di-tert-butylphenol (2,4-DTBP)
O Takahashi et al.
Xenobiotica; the fate of foreign compounds in biological systems, 13(5), 319-326 (1983-05-01)
Single oral doses of the haemorrhagic antioxidant 2,4,6-tri-t-butylphenol (260 mg/kg) were well absorbed in the rat. Peak blood levels of this compound were reached in 15-60 min. The blood elimination half-lives were 18.2 min for the alpha-phase and 11.8 h
Yoshinori Kadoma et al.
Chemosphere, 74(5), 626-632 (2008-12-17)
To clarify the mechanism of phenol toxicity, the radical-scavenging activity of 2- or 2,6-di-tert-butyl- and 2-methoxy-substituted phenols was investigated by combining two distinct approaches: first, the induction period method for methacrylate polymerization initiated by benzoyl peroxide or 2,2'-azobisisobutyronitrile; and secondly
A L Wilcox et al.
Chemical research in toxicology, 6(4), 413-416 (1993-07-01)
13-Hydroperoxyoctadeca-9,11,15-trienoic acid was reacted with a catalytic amount of 5,10,15,20-tetraphenyl-21H,23H-porphyrin iron(III) chloride in dichloromethane containing 2,4,6-tri-tert-butylphenol. The principal products were identified as 13-oxooctadeca-9,11,15-trienoic acid, 13-oxotrideca-9,11-dienoic acid, and a series of isomeric epoxyaryl ethers [9-(2,4,6-tri-tert-butylphenoxy)-12,13-epoxyoctadec-10-enoic acids and 11-(2,4,6-tri-tert-butylphenoxy)-12,13-epoxyoctadec-9-enoic acids]. The epoxyaryl
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.