모든 사진(1)
About This Item
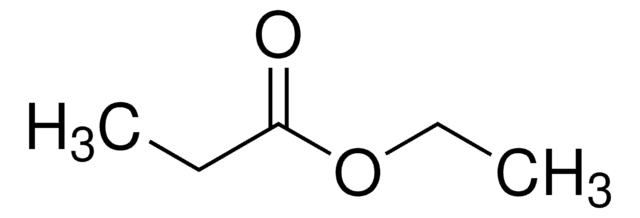
Linear Formula:
CH3COCH2COOC2H5
CAS Number:
Molecular Weight:
130.14
FEMA Number:
2415
Beilstein:
385838
EC Number:
유럽평의회 번호:
240
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
9.402
NACRES:
NA.21
감각 수용성의:
apple; fatty; green; fruity
Grade:
FG
Kosher
Kosher
생물학적 소스:
synthetic
Agency:
meets purity specifications of JECFA
식품 알레르기항원:
no known allergens
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Kosher
Agency
meets purity specifications of JECFA
규정 준수
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
vapor density
4.48 (vs air)
vapor pressure
1 mmHg ( 28.5 °C)
분석
≥99%
autoignition temp.
580 °F
expl. lim.
9.5 %
refractive index
n20/D 1.418-1.421
bp
181 °C (lit.)
mp
−43 °C (lit.)
solubility
water: soluble 130 g/L at 20 °C
density
1.029 g/mL at 20 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
감각 수용성의
apple; fatty; green; fruity
SMILES string
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- Fabrication of a novel magnetic nanostructure based on cellulose-gellan gum hydrogel, embedded with MgAl LDH as an efficient catalyst for the synthesis of polyhydroquinoline derivatives.: This study explores the use of ethyl acetoacetate in the synthesis of polyhydroquinoline derivatives, showcasing its application in developing efficient catalytic systems for organic reactions (Hjazi A, 2024).
- Evaluation of diethyl 4-(5-bromo-1H-indol-3-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate: synthesis, anti-corrosion potential, and biomedical applications.: This research investigates the biomedical applications and anti-corrosion properties of compounds synthesized using ethyl acetoacetate, emphasizing its versatility in chemical synthesis and material science (Ahamed FMM et al., 2024).
- Fe(3)O(4) nanoparticles impregnated eggshell as an efficient biocatalyst for eco-friendly synthesis of 2-amino thiophene derivatives.: The study highlights the use of ethyl acetoacetate in green chemistry, particularly in the eco-friendly synthesis of thiophene derivatives using biocatalysts (Zargari M et al., 2024).
- Pyrano[2,3-c]pyrazole fused spirooxindole-linked 1,2,3-triazoles as antioxidant agents: Exploring their utility in the development of antidiabetic drugs via inhibition of α-amylase and DPP4 activity.: This paper discusses the synthesis of novel compounds with antidiabetic properties using ethyl acetoacetate, demonstrating its potential in drug development (Chahal S et al., 2024).
- Access to Functionalized Cyclohex-2-enones from a Multicomponent Cascade Reaction of Readily Available Alkynes, Ketones, and Ethyl Acetoacetate.: The research details a multicomponent cascade reaction involving ethyl acetoacetate, highlighting its utility in the efficient synthesis of functionalized cyclohexenones (Jiang D et al., 2024).
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
164.3 °F - closed cup
Flash Point (°C)
73.5 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Junguo Xin et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(10), 3177-3181 (2008-02-05)
An enantioselective Biginelli reaction that proceeds by a dual-activation route has been developed by using a combined catalyst of a readily available trans-4-hydroxyproline-derived secondary amine and a Brønsted acid. Aromatic, heteroaromatic, and fused-ring aldehydes were found to be suitable substrates
Jer Yiing Houng et al.
Journal of biotechnology, 100(3), 239-250 (2002-11-22)
This study examined the characteristics and operational parameters of the asymmetric reduction of ethyl 4-chloro acetoacetate by bakers' yeast in order to produce S-4-chloro-3-hydroxybutyric acid ethyl ester. Eight operational variables were also optimized using the Taguchi method with consideration of
Gui-Rong Qu et al.
Organic letters, 11(8), 1745-1748 (2009-03-20)
A novel approach to the synthesis of purines bearing functionalized carbon substituents or methyl in position 6 was developed. Under different reaction conditions, 6-halopurine derivatives could react with ethyl acetoacetate efficiently to yield 2-(purin-6-yl)acetoacetic acid ethyl esters, (purin-6-yl)acetates and 6-methylpurines
Hassan Valizadeh et al.
Molecular diversity, 15(1), 233-237 (2010-07-24)
In this study, a three-component one-pot synthesis of select 5-amino-6-cyano-3-hydroxybenzo[c]coumarin compounds derived from salicylaldehydes, malononitrile, and ethyl acetoacetate is reported. The reaction is conducted on grinding over MgO at room temperature resulting in good yields.
Arne T Dickschat et al.
Chemical communications (Cambridge, England), 49(22), 2195-2197 (2013-02-09)
Bifunctional mesoporous silica nanoparticles (MSNs) bearing Pd-complexes and additional basic sites were prepared and tested as cooperative active catalysts in the Tsuji-Trost allylation of ethyl acetoacetate. Functionalization of the MSNs was realized by postmodification using click-chemistry. The selectivity of mono
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.